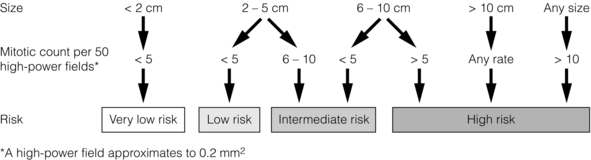
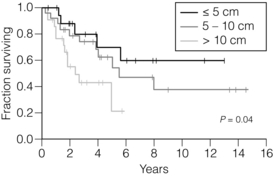
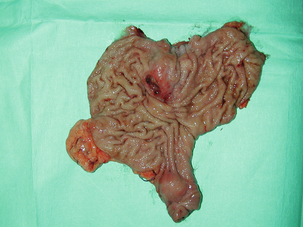
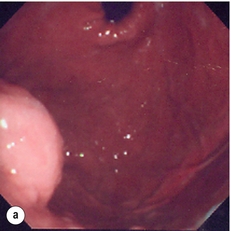
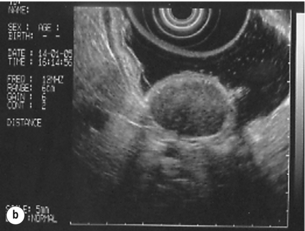
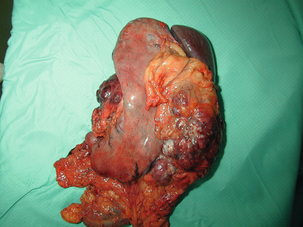
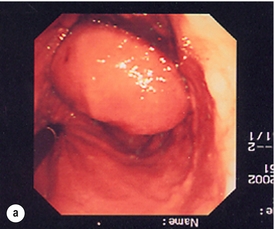
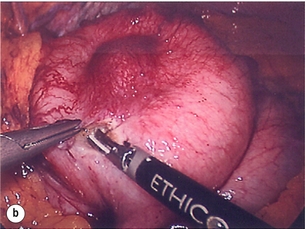
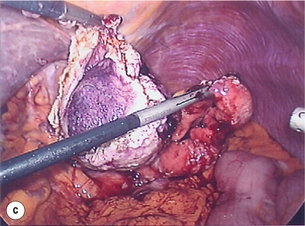
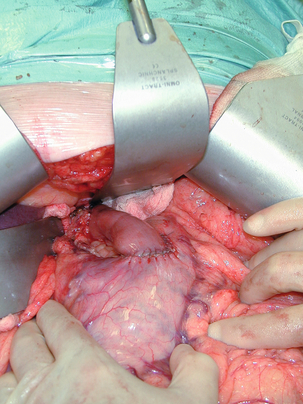
11 GISTs are soft-tissue sarcomas of mesenchymal origin that arise in the gastrointestinal tract; they are rare, representing 0.1–3% of all gut tumours and 5% of all soft-tissue sarcomas.1 Historically, these tumours were considered to be of smooth muscle origin and were generally regarded as leiomyomas (benign) or leiomyosarcomas (malignant). Electron microscopy and immunohistochemical studies indicated, however, that only a minority of stromal tumours have the typical features of smooth muscle, with some having a more neural appearance and others appearing undifferentiated.2 ‘Gastrointestinal stromal tumour’ was subsequently introduced as being a more appropriate term for these neoplasms, with the variable histological features (smooth muscle, neural or undifferentiated) considered to be of little clinical relevance. Gastrointestinal autonomic nerve tumour (GANT) was also introduced to describe sarcomas with ultrastructural evidence of autonomic nervous system differentiation,3 but these tumours are now recognised as a variant of GIST.4 The discovery of CD34 expression in many GISTs suggested that they were a specific entity,5 distinct from smooth muscle tumours. It was also observed that GISTs and the interstitial cells of Cajal (ICCs) express the receptor tyrosine kinase KIT (CD117).6 This has led to the now widely accepted classification of mesenchymal tumours of the gastrointestinal (GI) tract into GISTs, true smooth muscle tumours and, far less frequently, true Schwann cell tumours.7 GISTs are microscopically classified into three histological subtypes: spindle cell (70%), epithelioid (20%) and mixed (10%). Immunohistochemically, more than 90% of GISTs stain positive for CD117. A new antibody, DOG-1, is also highly sensitive and specific for GISTs.8 The commonest sites of mutations in the c-kit gene are in exon 11 (60–70%), followed by exon 9 (18%), and exons 13 and 17 (3%). It is important that an experienced pathologist examines the immunohistochemical profile of any mesenchymal tumour as CD117-positive staining can be seen in other tumours such as seminomas, small-cell lung cancer, thyroid cancer and melanomas. Studies using diagnostic markers including CD117 immunoreactivity have shown that GISTs are under-diagnosed.6 The morphological spectrum of GISTs was also wider than previously recognised. The estimated annual incidence of GISTs is around 15 per million,9 which equates to approximately 900 new cases per year in the UK. A true measure of the incidence, prevalence and ratio of ‘benign’ to ‘malignant’ GISTs may not be possible as these tumours appear to possess varying degrees of malignant potential. The size of the tumour, the symptoms at diagnosis, the organ of origin (small-bowel GISTs have the worst prognosis) and mitotic count seem to be the most important factors when assessing prognosis.10 No marked sex difference is apparent for GISTs. Two larger series of malignant GI sarcomas did, however, demonstrate a slight male predominance.12,13 The age distribution appears to be unimodal, with a median age at presentation of 58 years (range 16–94). The peak incidence in men occurs in the fifth decade, slightly before that in women, where it peaks in the sixth decade. The median age at presentation appears constant in several series, ranging from 58 to 61 years.14 Only 1–2% of GISTs present in patients before 30 years of age.12 Most GISTs arise in the stomach or small intestine, and infrequently in the oesophagus, mesentery, omentum, colon or rectum13,15 (Table 11.1). Approximately 10–30% of GISTs are overtly malignant at presentation;16 the principal sites of metastasis are the liver and the peritoneal cavity, and spread to lymph nodes is very rare.12 Table 11.1 The symptoms of GISTs are non-specific and depend on the size and location of the lesion. Small GISTs (2 cm or less) are usually asymptomatic and are detected during investigations or surgical procedures for unrelated disease. The vast majority of these are of low risk for malignancy.17 In many cases the mucosa is normal so that endoscopic biopsies are unremarkable. Incidental discovery accounts for approximately one-third of cases.18 The most common symptom is GI bleeding, which is present in approximately 50% of patients19 (Table 11.2). In addition, systemic symptoms such as fever, night sweats and weight loss are common in GIST and rare in other sarcomas. Patients with larger tumours may experience abdominal discomfort or develop a palpable mass.20 GISTs are often clinically silent until they reach a large size, bleed or rupture. Symptomatic oesophageal GISTs, although rare, typically present with dysphagia, while gastric and small-intestinal GISTs often present with vague symptoms leading to their eventual detection by gastroscopy or radiology. Most duodenal GISTs occur in the second part of the duodenum, where they push or infiltrate into the pancreas.21 Table 11.2 Symptoms of GIST at diagnosis19 Approximately 60% of GISTs are submucosal and grow towards the lumen where, if in the proximal GI tract, they may be visualised endoscopically as smooth submucosal projections. If a small submucosal mass is seen as an incidental finding at the time of endoscopy, endoscopic ultrasound (EUS) should be the first investigation, as a significant proportion will be due to extrinsic impression from normal adjacent structures, e.g. gall bladder in the antrum, and spleen in the proximal stomach. If this is the case, no further investigation is required. For larger palpable masses, or where the patients present with haemorrhage, abdominal pain or obstruction, computed tomography (CT) is usually the first investigation after endoscopy to both assess the primary and look for metastases.22 The classical features are of a hypoechoic mass contiguous with the fourth (muscularis propria) or second (muscularis mucosae) layers of the normal gut wall, both of which are hypoechoic (Fig. 11.3a,b). The EUS features most predictive of ‘benign’ tumours are regular margins, tumour size ≤ 30 mm and a homogeneous echo pattern. Larger tumours with irregular extraluminal margins and cystic spaces are more likely to behave aggressively.23,24 Figure 11.3 (a) Endoscopic view of a small incidental gastric GIST. (b) A 12-MHz EUS image of the incidental gastric GIST seen in (a), showing the lesion arising from the muscularis propria. To further aid diagnostic accuracy it is possible to use a linear EUS scope through which needle aspirates and core biopsies can be taken without breaching surgical resection planes. EUS with fine-needle aspiration (EUS-FNA) in experienced hands has a diagnostic accuracy of up to 97% for GIST lesions,25 is becoming more widely available and should be considered in the diagnostic work-up of a possible GIST lesion if the result could change clinical management. GIST imaging by CT typically shows an extraluminal mass, often with central necrosis, arising from the digestive tract wall.18 Small tumours typically appear as sharply margined, smooth-walled, homogeneous, soft-tissue masses with moderate contrast enhancement.26 Large tumours tend to have mucosal ulceration, central necrosis and cavitation, and heterogeneous enhancement following i.v. contrast.26 As well as defining the presence and nature of a mass, if possible, the likely organ of origin should be defined. Multiplanar reconstruction can assist this, particularly with large masses. Negative oral contrast (e.g. water) and intravenous contrast for the assessment of gastric GISTs is recommended. CT of chest, abdomen and pelvis is recommended for staging of GIST, with the exception of small incidental tumours or when a patient presents as an emergency requiring urgent surgery. With regards to assessing treatment response, traditional CT criteria (RECIST criteria) have been shown to be inaccurate for measuring GIST response to imatinib and the Choi criteria are recommended (10% reduction in size and 15% reduction in density).27 In general, MRI offers no additional information regarding the intralesional tissue characterisation of primary GISTs. However, MRI provides excellent soft-tissue contrast resolution and direct multiplanar imaging, which can help delineate the relationships of the tumour and adjacent organs, and is useful in anorectal disease.26 PET scanning using a standard fluorodeoxyglucose (FDG)-PET technique has proven extremely useful in the prediction of tumour response to the tyrosine kinase inhibitor imatinib (Glivec, Norvartis Pharma AG) now used in the treatment of unresectable and metastatic malignant GISTs.28 Glucose uptake of the tumours decreases within a few hours to days of the start of treatment, which can be verified with FDG-PET.17 The PET scan can be utilised to distinguish between tumour progression and increase in volume due to intratumoral bleeding. PET scan responses have also been demonstrated to predict subsequent tumour volume reductions found on CT or MRI.29 Families have been reported with single-base ‘gain of function’ mutation in the kinase domain of KIT. The resultant effect is the development of multiple GISTs in the small bowel. Diffuse hyperplasia of spindle-shaped cells within the myenteric plexus at sites unaffected by GIST formation was also noted.30,31 The association of three uncommon neoplasms – gastric GIST, functioning extra-adrenal paraganglionoma and pulmonary chondroma – was first reported in 1977 and has since been recognised as ‘Carney’s triad’ (Fig. 11.4).32 A subsequent review of 79 cases demonstrated that, unlike isolated sporadic GIST, where no significant sex difference was noted, 85% were female.33 Twenty-two per cent of the patients had all three tumours; the remainder had two of the three, usually the gastric and pulmonary lesions. Adrenocortical adenoma has since been identified as a new constituent of the disorder. The presence of two of the three main tumours is considered sufficient for the syndrome. Percutaneous (ultrasound or CT) or laparoscopically guided biopsies should not be used in resectable disease due to the risk of tumour rupture or seeding, unless it may result in a change of treatment.17 Laparoscopy may be considered in the staging of large lesions to exclude peritoneal metastases but an exploratory laparotomy is usually required to decide whether a large primary tumour is technically resectable or not. At all sites the extent of resection is therefore dictated by the size of the tumour and its location in relation to, or invasion of, adjacent structures (Fig. 11.5). Oesophagectomy is the standard procedure for oesophageal GISTs but these are very rare and submucosal lesions in the oesophagus are much more likely to be leiomyomas. EUS-FNA core biopsy from these lesions is recommended to make a preoperative diagnosis so that surgical planning is appropriate.25 Oesophageal GISTs and leiomyosarcomas require an oesophagectomy whereas leiomyomas can safely be enucleated without removing the oesophagus. Figure 11.5 Operative specimen following en bloc total gastrectomy, splenectomy and distal pancreatectomy for locally advanced GIST. In the stomach, R0 resection may involve a partial, subtotal or total gastrectomy, although ‘wedge’ excision and ‘sleeve’ resections are also frequently performed to preserve as much stomach as possible. Small gastric lesions lend themselves well to laparoscopic resection (Fig. 11.6a–c). Resection of GIST tumours arising at the gastro-oesophageal junction creates particular problems as a poor quality of life may result from simple excision with anastomosis of stomach to oesophagus. Alternatively, reconstruction using a short jejunal interposition should be considered for these patients, the ‘Merendino procedure’ (Fig. 11.7),36 as it results in a better quality of life compared to an oesophagogastric anastomosis.37 The most important factors, as stated, are that the tumour is not ruptured and that negative resection margins are obtained. Simple enucleation of the tumour is inadequate as these lesions do not possess a true capsule. Direct invasion of adjacent structures occurs in 10–15% of GISTs and surgery in such cases should include en bloc resection of involved adjacent organs.12,14 Nodal metastases are extremely rare and routine extended lymph node dissection is therefore unjustified.38 Figure 11.6 (a) Endoscopic view of a moderate-sized gastric fundal GIST. (b) Laparoscopic image of the lesion seen in (a). (c) Completed harmonic scalpel dissection of the gastric GIST in (a) prior to removal of specimen in a retrieval bag and closure of the resulting gastric defect with a linear EndoGIA stapler. Figure 11.7 Completed Merendino procedure showing distal anastomosis between the jejunal interposition and the stomach. As very few studies address the issue of GISTs found incidentally, there are no clear data to support one definitive management plan over another. In their study of 39 GISTs, which included 16 identified incidentally, Ludwig and Traverso concluded that as a consequence of the frequency of serious complications in symptomatic patients, complete excision should also be recommended for asymptomatic patients.39 However, the UK guidelines for the management of GISTs recommend that small asymptomatic incidental lesions can be treated conservatively, particularly if serial examination shows no change in size over 1–2 years.40 However, there are no long-term studies of the natural history of these lesions, and surgeons should explain these uncertainties to their patients and discuss the pros and cons of resection before proceeding to surgery. In patients with borderline fitness for resection, or those who decline surgery at initial presentation, monitoring the lesion with EUS and/or CT for evidence of enlargement is acceptable so long as the results of surveillance influence the final management. Imatinib mesylate is a receptor tyrosine kinase inhibitor that inhibits the constitutively activated tyrosine kinases of ABL (including the stable transfection product fusion kinase BCR-ABL seen in chronic myeloid leukaemia), platelet-derived growth factor receptor (PDGFR) and KIT. The drug is administered orally, and its use, dosage and side-effect profile are well established following use in the treatment of chronic myeloid leukaemia. It has very little effect on normal cells, where the kinase is not constitutively active. Experiments on human tumour cell lines dependent upon the KIT pathway demonstrate that imatinib blocks the kinase activity of KIT, arrests proliferation and causes apoptotic cell death.41 Imatinib is generally well tolerated, although most patients experience some mild or moderate adverse events. Serious adverse events occur in around 20% of patients, the most serious of which is life-threatening tumour haemorrhage in approximately 5%.
Other oesophageal and gastric neoplasms
Gastrointestinal stromal tumours (GISTs)
Incidence and malignant potential
Patient demographics and anatomical distribution
Site
Percentage
Stomach
60–70%
Small intestine
20–30%
Oesophagus, mesentery, omentum, colon or rectum
10%
Presentation
Symptoms
Incidence
Abdominal pain
20–50%
Gastrointestinal bleeding
50%
Gastrointestinal obstruction
10–30%
Asymptomatic
20%
Investigation
Endoscopic ultrasound (EUS)
CT scanning
Magnetic resonance imaging (MRI)
Positron emission tomography (PET)
GIST syndromes
Treatment and prognosis (Box 11.1)
Imatinib
![]()
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


Other oesophageal and gastric neoplasms