Fig. 6.1
The potential role of mesalamine in the current treatment algorithm for UC
In the present chapter, we will review the pharmacology, mechanisms of action, and effectiveness of oral mesalamine products in the management of UC.
5-Aminosalicylic Acid Formulations
There are currently various 5-aminosalicylic acid (5-ASA [mesalamine]) formulations (sulfasalazine, olsalazine, balsalazide, and mesalamine) available that utilize different methods to increase efficient delivery of the active ingredient to small and/or large intestine [19, 20]. Sulfasalazine was one of the first drugs that were introduced to treat UC. Earlier in the past century, sulfasalazine was started being used to treat rheumatoid arthritis. Swedish investigators initially found that sulfonamides were effective in treating septic arthritis and tried it in rheumatoid arthritis without success. They then coupled sulfonamide with salicylic acid, which was already shown to be effective in treating arthritis, under the assumption that it would actively carry the latter to the inflamed joints. This combination was not shown to be effective for rheumatoid arthritis, but they went on to try a variety of different combinations. One such combination was sulfasalazine, consisting of 5-ASA and sulfapyridine joined together by a diazo-bond. This proved to be effective in treating rheumatoid arthritis, and it then became widely used. Sulfapyridine, which is an antimicrobacterial drug directed against Gram-positive and Gram-negative intestinal bacteria, was already being used to treat UC, but then some physicians used sulfasalazine, and it turned out that it showed a great success in some cases with UC. The use of sulfasalazine gradually increased after the Second World War, and then in 1962, Baron et al. reported the results of a controlled, double-blinded study [21]. They showed that sulfasalazine was effective in the treatment of active UC and later also in maintaining remission of UC [22]. In rheumatoid arthritis, either 5-ASA or sulfapyridine, when given alone, is unlikely to show clinical benefit. 5-ASA is rapidly metabolized and secreted into urine once absorbed from the gastrointestinal tract. Contrary to its effect in inflammatory bowel diseases, studies suggest that the intact sulfasalazine molecule may possess anti-inflammatory properties in rheumatoid arthritis. Furthermore, it is known that about 10–20 % of orally administered sulfasalazine is absorbed systemically and can accumulate in connective tissues of inflamed joints, where it slowly releases 5-ASA, raising the possibility that it also acts as a prodrug in rheumatoid arthritis.
Research into the mechanisms of action of sulfasalazine revealed that it acted as a prodrug, with sulfapyridine working as a carrier and delivering the active component 5-ASA to the colon [23] (Fig. 6.2a). This also led to the discovery that sulfapyridine was responsible for the majority of adverse effects, such as headache, nausea, infertility, hemolytic anemia, and photosensitization. The development of novel drug delivery systems allowed direct delivery of the active moiety, 5-ASA, to the small bowel and colon. This can be broadly categorized into three groups [24]. The first are those that bind 5-ASA to another carrier, similar to sulfasalazine, which requires splitting of the diazo-bond by the colonic bacterial flora. The other two coat 5-ASA into either a pH-dependent formulation or a microsphere formulation. The oral mesalamine products currently available in the USA are summarized in Table 6.1.
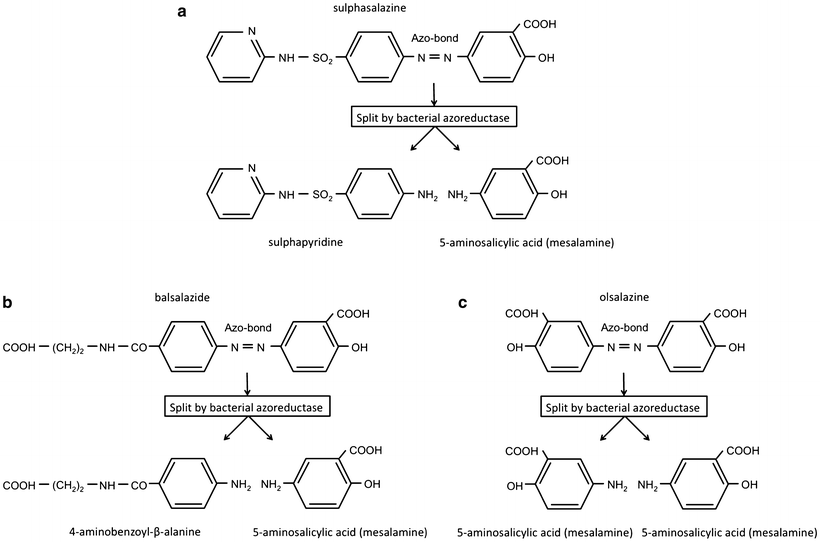

Fig. 6.2
(a) Chemical structure of sulfasalazine and its degradation to sulfapyridine and 5-aminosalicylic acid (mesalamine). (b) Chemical structure of balsalazide. (c) Chemical structure of olsalazine
Table 6.1
Currently available mesalamine formulations in the USA
Drug name | Active or prodrug | Characteristics and delivery method | Target site of release | Available tablet | Daily dosage | Dosing interval | Reference | ||
|---|---|---|---|---|---|---|---|---|---|
Generic | Trade | Active UC | Maintenance | ||||||
Sulfasalazine | Azulfidine® Azulfidine EN® | Prodrug | Uses sulfapyridine as a carrier and is degraded by bacterial azoreductase | Colon | 500 mg (200 mg 5-ASA) | 2–6 g | 2–4 g | tid | |
Balsalazide | Colazal® | Prodrug | Uses 4-aminobenzoyl-β-alanine as a carrier and degraded by bacterial azoreductase | Colon | 750 mg (262 mg 5-ASA) | 2–6.75 g | 2–6.75 g | tid | |
Olsalazine | Dipentum® | Prodrug | Dimer of 5-ASA and is degraded by bacterial azoreductase | Colon | 250 mg | 2–3 g | 1 g | bid | |
Mesalamine (5-ASA) | Pentasa® | Active | Ethyl cellulose coated and controlled release throughout the small bowel and colon | Duodenum-colon | 250, 500, 1000 mg | 2–4 g | 2–4 g | qid | |
Delzicol® Asacol HD® | Active | Eudragit-S coated and pH-dependent release at pH ≥ 7 | Terminal ileum-colon | 400, 800 mg | 1.6–4.8 g | 0.8–4.8 g | tid | ||
Apriso® | Active | Eudragit-L delayed and extended release at pH ≥ 6 | Jejunum-colon | 500 mg | 1.5–4.5 g | 1.5 g | qd | ||
Lialda® | Active | MMX® delivery and released at pH ≥ 7 | Colon | 1,200 mg | 2.4–4.8 g | 2.4 g | qd | ||
Prodrugs
Sulfasalazine, balsalazide, and olsalazine work as a prodrug and use a similar mechanism to carry the active moiety, 5-ASA, to the colon. As mentioned above, sulfasalazine consists of 5-ASA and sulfapyridine joined together by a diazo-bond. Sulfasalazine is 40 % 5-ASA, and about 80–90 % reaches the colon, where it is broken down to 5-ASA and sulfapyridine by the azoreductase of the colonic microbiota [23]. The bioavailability of the 5-ASA moiety that is released ranges from 11 to 33 %. Balsalazide instead uses 4-aminobenzoyl-β-alanine as a carrier to reach the colon [25]; 4-aminobenzoyl-β-alanine and 5-ASA are joined by a diazo-bond (Fig. 6.2b). Balsalazide is 35 % 5-ASA, and nearly the entire dose reaches the colon, where the diazo-bond is uncleaved to release 5-ASA. The bioavailability for balsalazide ranges from 12 to 35 % [26]. Olsalazine is a polymer of two molecules of 5-ASA joined together by a diazo-bond, and it contains about 89 % of 5-ASA [27, 28] (Fig. 6.2c). Approximately 98 % reaches the colon, where it releases two molecules of 5-ASA. The bioavailability of the 5-ASA moiety that is released is about 14–31 %. These three products are all delivered to the colon as an intact form (~about 90–99 %) and are degraded by the bacterial azoreductase to release 5-ASA. Azoreductase is an intracellular enzyme possessed mainly by a wide range of colonic bacteria, which rapidly uncleaves the diazo-bond. Concurrent use of antibiotics that affect the colonic microbiota can decrease the metabolism of these three agents, as can shortened colonic transit time, such as diarrhea, medication, and colectomy. Some studies suggest that during active inflammatory bowel disease, the bioavailability of the bacterial enzymes including azoreductase are compromised, which may interfere with its clinical efficacy [29].
pH-Dependent Formulations
pH-dependent formulations utilize the gradient of pH in the gastrointestinal tract to deliver the active agent into aimed part of the intestine. The pH in the stomach is approximately 2. The pH in the upper small bowel is about 5–6, and in the lower parts of the small bowel including the terminal ileum, it reaches 6–7. Throughout the colon, the pH is maintained close to neutral between 7 and 8 [30]. Asacol® utilizes an acrylic-based resin (Eudragit-S) coating that is soluble at a pH of >7, thus delivering 5-ASA in the terminal ileum and entire colon [31]. Asacol® was recently replaced by Delzicol®, which is a bioequivalent product that does not contain dibutyl phthalate (DBP), a solvent with potential adverse effect on fetal reproductive system [32, 33]. Lialda® is a Multi-Matrix System (MMX®) mesalamine (marketed as Lialda® in the USA and Mezavant® in the European Union) that is designed to deliver 5-ASA throughout the entire colon as a high-strength once-daily dosing tablet [34, 35]. The mechanism of the MMX® delivery system is a double matrix consisting of a lipophilic matrix dispersed within a hydrophilic matrix [36]. 5-ASA is incorporated into microparticles in the lipophilic matrix, contained within the hydrophilic matrix. This double matrices is then covered by a pH-dependent polymer film that delays the release of 5-ASA until the film dissolves when exposed to a pH of >7.0 in the terminal ileum to colon. When the hydrophilic matrix is exposed to the intestinal fluids, it swells and creates a viscous gel mass, theoretically resulting in slow dispersion of 5-ASA. The hydrophilic matrix adheres to the colonic mucosa, which also contributes to targeted drug delivery to the colon. Apriso® utilizes a patented delivery system called IntellicorTM delayed- and extended-release delivery system that provides coverage throughout the colon [37]. It disintegrates at pH 6.0 in the distal jejunum where the 5-ASA begins to be released. This formulation combines delayed and sustained release and allows the5-ASA to travel through the jejunum to the colon. It provides the convenience of once-daily dosing like the MMX® mesalamines.
Microsphere Formulation
Pentasa® is currently the only available microsphere formulation of mesalamine [38, 39]. It encapsulates 5-ASA in ethyl cellulose-coated microgranules that gradually starts to release 5-ASA beginning in the duodenum. The release of 5-ASA from the microgranules is not affected by the bowel acidity and occurs in any enteral pH conditions. Thus, the release continues throughout the jejunum, the ileum, and the colon as well as the rectum.
Mechanisms of Action of Mesalamine
Aspirin and nonsteroidal anti-inflammatory drugs (NSAIDs) have been shown to inhibit prostaglandin synthesis by blocking the effect of cyclooxygenase (COX) enzymes. Similarly to aspirin, its breakdown product salicylate suppresses local prostanoid production at sites of inflammation. 5-ASAs, i.e., mesalamines, are one of the most widely used salicylates, but its pharmacological profile and mechanism of action remains to be fully elucidated. In 1977, Khan et al. demonstrated in a non-placebo-controlled trial that 5-ASA is the active therapeutic moiety of sulfasalazine. Free 5-ASA, if administered orally, is rapidly absorbed from the upper intestine. 5-ASA is poorly absorbed from the colonic mucosa, and about 50 % will be metabolized to acetyl-5-ASA by the intestinal epithelium and luminal bacteria [40]. The absorbed 5-ASA is also metabolized to acetyl-5-ASA in the liver and then excreted into the urine as a mixture of free 5-ASA and acetyl-5ASA. Acetyl 5-ASA is therapeutically inactive, and it is presumed that 5-ASA acts topically on the mucosa of the gastrointestinal tract. This led to the subsequent development of various mesalamine products. After oral or rectal administration into the colon, small amounts of mesalamine is absorbed by the intestinal epithelial cells, but most are passed into the stool in an intact form [40, 41]. The mucosal concentrations of 5-ASA ranged from 3 to 50 ng/mg of wet colonic tissues in patients receiving standard treatment with mesalamine [23]. The therapeutic effect of 5-ASA is dependent on the direct contact of the molecule with the epithelial cells of the intestine than on its tissue concentration, which suggests that a high intraluminal concentration of 5-ASA is required for its action. The proposed mechanisms regarding the effect of mesalamine are summarized in Table 6.2.
Table 6.2
Proposed mechanism of action of mesalamine
Proposed mechanism | Reference |
|---|---|
Blocking the production of prostaglandin and leukotrienes | |
Inhibition of pro-inflammatory cytokine production | |
Inhibition of iNO | [50] |
Free radical scavenger and antioxidant effect | |
Inhibition of the activation of NF-KB | |
Increasing PPAR-γ expression in epithelial cells | [49] |
Sulfasalazine and mesalamine inhibit the COX and lipoxygenase pathways resulting in reduced production of prostaglandins and leukotrienes, respectively [42, 43]. Prostaglandins and leukotrienes are chemotactic and pro-inflammatory factors that play a major role in the inflammation of inflammatory bowel disease [44, 45], and the anti-inflammatory effect of mesalamine is in part by the effects on their metabolism [46, 47]. Mesalamine also inhibited the transcription of inflammatory mediators in intestinal epithelial cells, which counteracted the antiproliferative effects of TNF-α [48]. Several studies have shown that mesalamine inhibited the production of pro-inflammatory cytokines including interleukin-1 (IL-1) from colonic epithelial cell lines [49, 50]. Egan et al. demonstrated that mesalamine modulated RelA/p65 phosphorylation which ultimately decreased transcriptional activity of NF-κB [51]. Mesalamine also suppressed TNF-α activation of NF-κB by inhibiting the TNF-α-stimulated NF-κB inhibitory protein kinase α (IKKα) activity toward IκBα in intestinal epithelial cells [48, 52]. Peroxisome-proliferator-activated receptor-γ (PPAR-γ) are members of the nuclear receptor superfamily, which are activated by fatty acids. They are involved in the transduction of metabolic and nutritional signals into transcriptional responses. Rousseaux et al. showed that mesalamine increased PPAR-γ expression in epithelial cells. The translocation of PPAR-γ from the cytoplasm to the nucleus was enhanced and resulted in the activation of a peroxisome-proliferator response element-driven gene. These results were likely responsible for the therapeutic effect of mesalamine on colitis induced in wild type, but not PPAR-γ+/− mice [53]. Nitric oxide (NO) is an important final effector of mucosal injury in inflammatory bowel disease. Kennedy et al. showed that mesalamine inhibited inducible NO (iNO) production by human intestinal epithelial cells lines [54]. This was owing to the mesalamine-induced inhibition of the expression of iNO synthetase (iNOS) protein and mRNA and the suppression of cytokine-induced transcriptional upregulation of the iNOS gene. Various studies have also shown that mesalamine prevents tissue damage caused by neutrophil-derived oxidants [55–58]. Greenfield et al. showed that sulfasalazine suppressed the upregulation of HLA molecules on leucocytes, suggesting an immunological effect of mesalamines [59].
Adverse Effects of Mesalamine
The clinical efficacy of sulfasalazine is dose related; however, not many can tolerate the drug at higher doses due to side effects. The incidence of side effects from sulfasalazine is reported to be about 45 % [40]. The side effect profile of sulfasalazine includes those that are unique to the compound and others, which are common to all mesalamine products. Most of the side effects are intolerance, not allergy, and are related to the sulfapyridine moiety. These include nausea, vomiting, and headache. Symptoms usually occur soon after initiation of sulfasalazine therapy in those patients who are taking higher doses. More severe reactions are uncommon but include allergic responses, various skin eruptions (urticaria, photosensitivity, maculopapular lesions, and epidermal necrolysis), pancreatitis, pulmonary reactions (bronchiolitis obliterans with organizing pneumonia, and eosinophilic pneumonitis, and pleuritis), hepatotoxicity (transaminitis, cholestasis), and arthralgias. Hematologic side effects such as agranulocytosis and immune thrombocytopenia are generally related to the sulfapyridine moiety. Spermatogenic dysfunction such as abnormal sperm counts, motility, and morphology that may contribute to reversible male infertility have also been attributed to sulfapyridine. Sulfasalazine inhibits folate absorption by way of competitive inhibition of folate conjugation. This may cause folate deficiency that hinders DNA synthesis and cell division, affecting most notably the bone marrow. When not many other mesalamine products were available, desensitization was used as a method to overcome allergic reactions to sulfasalazine [60, 61]. This was accomplished by starting at a very low dose of sulfasalazine and gradually increasing its dose after confirming the safeness until the desired dose is reached. This method can also be applied to mesalamine products [62].
Mesalamine is contraindicated in patients who have had hypersensitivity reactions to salicylates in the past. Those who were intolerant or had an allergic reaction with sulfasalazine may be able to take mesalamine without risk of similar reaction. However, of course, introduction of mesalamine should be done with caution in patients with a reported adverse event with sulfasalazine. Renal dysfunctions, including acute and chronic interstitial nephritis and minimal change nephropathy, can occur with sulfasalazine and mesalamine products. We recommend that all patients treated with mesalamine products should have their kidney function evaluated prior to the initiation of therapy and at least annually thereafter. Pulmonary and cardiac hypersensitivity reactions, such as pleuritis, pneumonitis, myocarditis, and pericarditis, have been reported with various mesalamine products. Other minor side effects include alopecia, abdominal distention and flatulence, headache, liver dysfunction, arthritis, skin changes, leucopenia, etc [40, 63, 64].
An acute intolerance syndrome characterized by abdominal pain, diarrhea, and fever may rarely occur with mesalamine therapy [65, 66]. It is difficult to distinguish between a flare of the underlying colitis; however, if intolerance syndrome is suspected, mesalamine should be discontinued immediately. Intolerance syndrome is universal to all mesalamine products, and patients require alternative treatments.
Drug Interactions
The risk of renal dysfunction may be increased in patients receiving known nephrotoxic agents, such as NSAIDs. Coadministration of azathioprine or mercaptopurine with mesalamine products may result in an increase in blood 6-thioguanine nucleotide concentrations which may lead to leucopenia [67].
Oral Mesalamine During Pregnancy and Lactation
When sulfasalazine and mesalamine are given orally, the absorbed mesalamine readily crosses the placenta, and mesalamine and its metabolite, acetyl-5ASA, are detected in the cord blood. However, this has not been linked to any fetal abnormalities in several large studies [68, 69]. Female patients taking sulfasalazine who are considering becoming pregnant should take folic acid to decrease the risk of neural tube defects. Animal studies in rodents have not revealed any evidence of impaired fertility or harm to the fetus.
Overall, sulfasalazine and mesalamine products are classified as Food and Drug Administration (FDA) pregnancy Category B, except for Asacol® that is categorized as C. The coating of Asacol® contains dibutyl phthalate (DBP) [70]. In animal studies, DBP was associated with external and skeletal malformations and adverse effects on the male reproductive system at doses >190 times the human dose. Though there are no adequate and well-controlled studies in pregnant women for either sulfasalazine or mesalamine, they can generally be continued safely during any trimester of pregnancy. Low concentrations of mesalamine and higher concentrations of the N-acetyl metabolite have been detected in human breast milk; however, in general, it can be used safely during lactation.
Therapeutic Efficacy of Mesalamine
Mesalamine for Active Mild to Moderate UC
The 5-ASA formulations (sulfasalazine, olsalazine, balsalazide, and mesalamine) have long been foundational treatments for mild to moderate UC. Guidelines suggest that combination of oral and topical therapies induces remission in mild to moderately active distal colitis patients and may effectively maintain remission [71–73]. As described above, the therapeutic effect of mesalamine in UC depends on the ability of the active drug to reach the sites of inflammation for topical (not systemic) anti-inflammatory activity. The currently available oral mesalamine preparations each utilize a slightly different method to increase efficient delivery of the active ingredient to small and/or large intestine. These methods include incorporation of mesalamine into a prodrug through covalent azo-bond, incorporation of unmodified mesalamine into a pH-sensitive acrylic coating or moisture-sensitive ethyl cellulose microspheres, and a newer formulation that utilizes both a pH-sensitive acrylic layer and a coating of lipophilic/hydrophilic excipients [19, 39, 74, 75]. All formulations are equally as effective, though the use of sulfasalazine is limited in its use mainly due to patient intolerance [76].
Dating back to the 1950–1960s, several studies demonstrated that sulfasalazine was superior to placebo for the treatment of active UC. In these initial studies, sulfasalazine was used at a dose of 4–6 g daily and was effective in 60–80 % of patients as compared to 30–40 % of the patients treated with placebo [21, 77, 78]. The response was also dose dependent.
Two randomized double-blinded studies demonstrated that balsalazide 6.75 g/day was as effective as sulfasalazine 3 g/day, but with a favorable safety profile [75, 79]. Balsalazide was shown to be superior to mesalamine in a double-blinded randomized trial for active UC. Patients on balsalazide 6.75 g/day showed superior 12-week clinical remission rate as compared to mesalamine 2.4 g/day (62 % vs. 37 %, p = 0.02), and it appears that the effect of balsalazide was more rapidly achieved [80]. Several other studies demonstrated similar efficacy between balsalazide and mesalamine [64]. In a double-blinded placebo-controlled trial, olsalazine 2 g/day was superior to placebo in achieving a clinical and endoscopic response [27]. Several studies have shown comparable efficacy between olsalazine 1.5–2 g/day and sulfasalazine 3 g/day for mildly to moderately active UC [81–83].
Asacol® was shown to be superior to placebo at a dose of 1.6 or 2.4 g/day in achieving remission at 6 weeks (43 % and 49 % vs. 23 % of placebo, p = 0.03, and 0.003, respectively) [84]. Schroeder et al. showed superior rates of remission with Asacol® 4.8 g/day compared to placebo (24 % vs. 5 %, p = 0.047) [5]. The rates of clinical response were more significant in patients with left-sided colitis (75 % vs. 21 %, p = 0.0001). Pentasa® was more effective than placebo at 2 or 4 g/day in achieving clinical and endoscopic remission at 8 weeks [85]. A double-blind trial between Pentasa® and sulfasalazine showed that Pentasa® at a dose 2.4 g/day was superior to sulfasalazine 2 g/day in achieving symptomatic and endoscopic improvement at 4 weeks [86].
In a dose-finding study of Apriso®, Kruis et al. demonstrated that doses of 1.5 g, 3 g, or 4.5 g daily were equally as effective for active UC [87]. There were no significant differences in remission rates, time to response, endoscopic improvement, or histological improvement. In another study, 3 g once-daily dosing was as effective as 1 g three times daily, suggesting that once-daily dosing of Apriso® is efficacious [88]. Lialda®, a once-daily mesalamine product, was tested in a randomized clinical trial of 2.4 g, 4.8 g, and placebo for 8 weeks [35]. Remission rates with both doses of Lialda® were significantly superior to placebo, but not different from each other.
More recently, the interest of mesalamine therapy has focused on optimized dosing and once-daily formulations.
High-Dose Mesalamine
The ASCEND (Assessing the Safety and Clinical Efficacy of a New Dose of 5-ASA) trials aimed to investigate the dose–response effect of mesalamine (Asacol®) in the induction of remission in UC. The ASCEND I trial randomized 301 patients with mildly to moderately active UC to receive either 2.4 g/day or 4.8 g/day of Asacol® [89]. At week 6, similar proportion of patients experienced improvement in each group (51 % vs. 56 %, p = ns). The difference was not significant; however, when results were stratified according to the disease severity, patients with moderate disease had a more substantial response to the 4.8 g/day dose compared to those with mild disease. Based on these results, the ASCEND II trial focused on moderately active UC and confirmed that 4.8 g/day of Asacol® led to a greater treatment response than 2.4 g/day (72 % vs. 59 %, p = 0.036) [90]. In the ASCEND III trial, 772 patients with moderately active UC were randomized to receive 2.4 g/day or 4.8 g/day doses of Asacol®, and there was no difference between the two groups in terms of overall improvement (complete remission and partial response), but significantly more patients who received 4.8 g/day compared to 2.4 g/day achieved clinical remission at week 3 (p = 0.02) and week 6 (p = 0.04) [74]. Furthermore, subgroup analysis showed that patients with difficult-to-treat disease, such as those previously treated with steroids, oral mesalamine, rectal therapies, or those taking multiple UC medications, responded better to higher doses than to lower doses.
Once-Daily Mesalamines
Studies have shown that only about half of the patients are adherent to multidose therapy [91, 92], and in clinical practice, poor adherence often leads to recurrence of disease [93]. Once-daily oral formulations may improve the adherence by decreasing the pill burden and, in fact, are a preferred choice by patients [94, 95]. Lialda® utilizes the MMX® system providing a slow and gradual release of mesalamine throughout the colon that permits once-daily administration [19]. Lichtenstein et al. performed a randomized, double-blind, parallel-group, placebo-controlled multicenter study in patients with mildly to moderately active UC comparing the effect of Lialda® 2.4 g/day given twice daily, 4.8 g/day given once daily, and placebo [96]. Similar proportion of patients achieved clinical remission with 2.4 g/day twice daily or 4.8 g/day once daily (34.1 % and 29.2 % vs. 12.9 % of placebo, p < 0.001 and p = 0.009, respectively). In another double-blind, placebo-controlled, multicenter trial, the effect of Lialda® 2.4 g once daily and 4.8 g once daily was compared to placebo and a delayed-release mesalamine (Asacol®) 2.4 g daily given three times daily [35]. At week 8, more patients achieved clinical and endoscopic remission in the Lialda® groups compared to placebo (40.5 % and 41.2 % vs. 22.1 %, vs. placebo; p = 0.01 and p = 0.007, respectively). No significant difference in clinical remission rates between patients receiving Asacol® and placebo was seen (33.7 % vs. 22.1 %, p = 0.089); however, it was unclear whether there was a statistical difference between Lialda® and Asacol®. Furthermore, a subsequent study combining the patients that achieved clinical and endoscopic remission in the abovementioned 2 studies evaluated the efficacy of Lialda® 2.4 g daily dosed once or twice daily as maintenance therapy [97]. At 12 months, similar proportion of patients was in clinical and endoscopic remission with once-daily and twice-daily regimen (64.4 % vs. 68.5 %, p = ns). Another once-daily mesalamine preparation (Salofalk®) that is available in Europe was evaluated in a dose-ranging trial and also proved to be efficacious in inducing remission in mildly to moderately active UC [98]. In a randomized, investigator-blinded study, controlled-release mesalamine (Pentasa®) was shown to be more effective when given in once-daily regimen [98]. At 12 months, 73.8 % of patients receiving Pentasa® 2 g once daily maintained remission compared to 63.6 % of patients receiving 1 g twice daily (p = 0.024). The study also demonstrated that compliance was better with once-daily regimen.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree






