Online Chapter 3
CASE 3.1 CLINICAL HISTORY 50-year-old woman with abdominal pain.
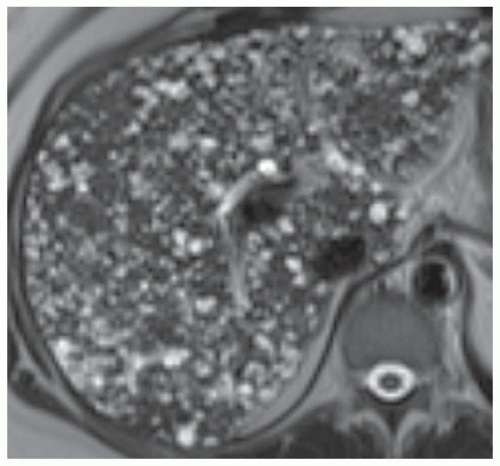
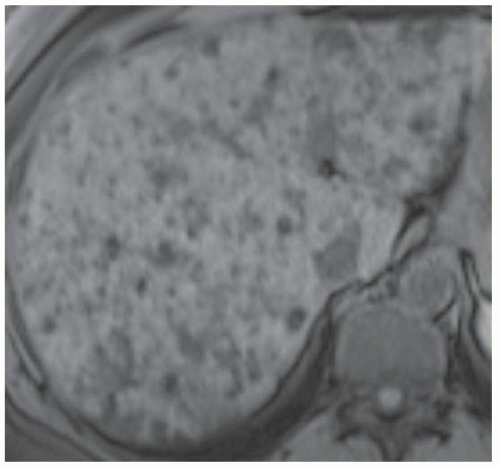
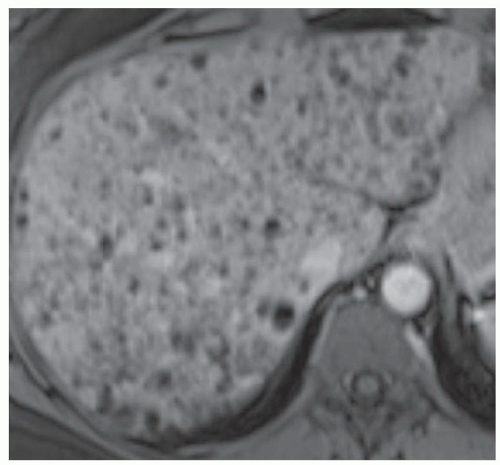
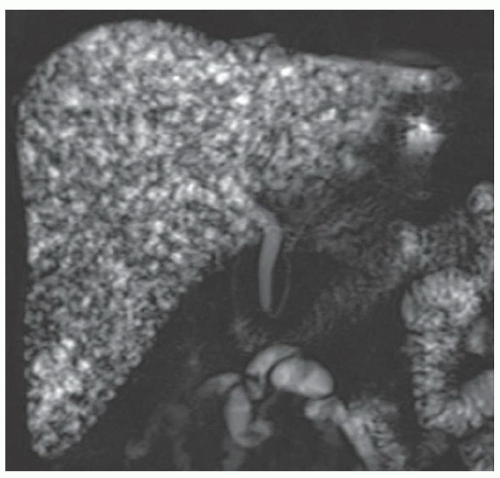
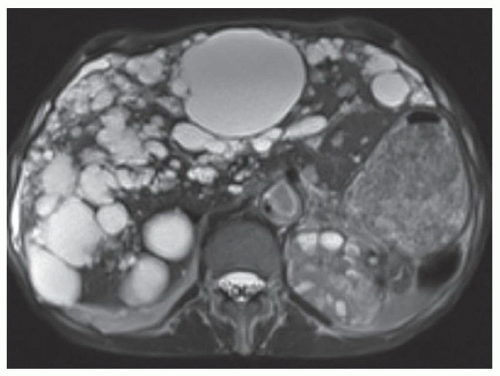
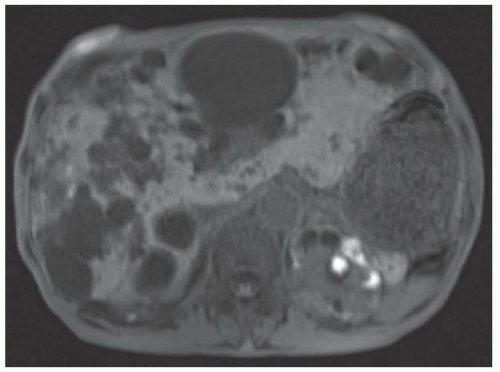
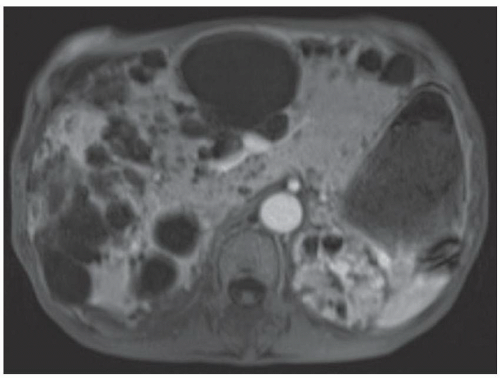
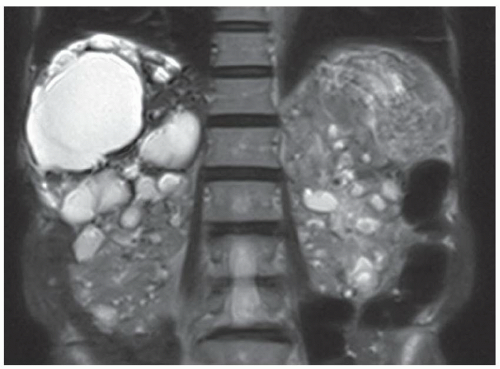
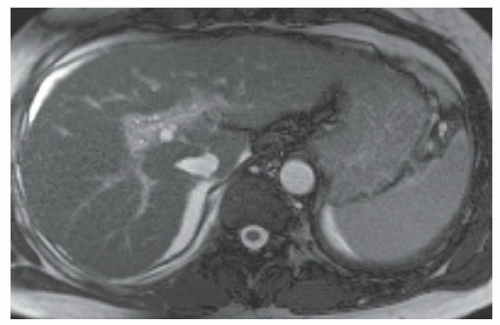
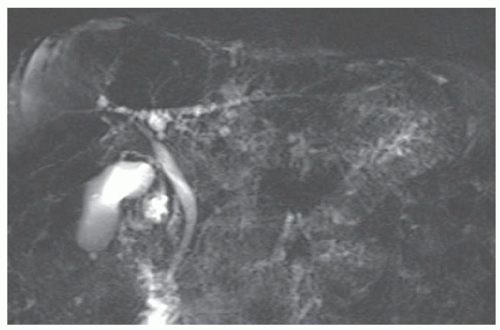
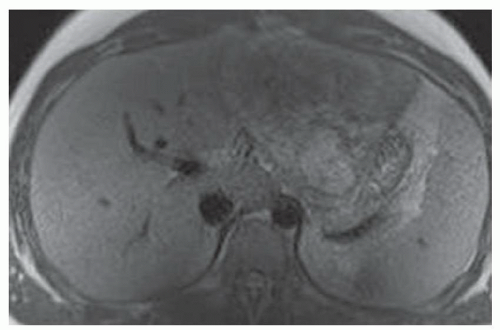
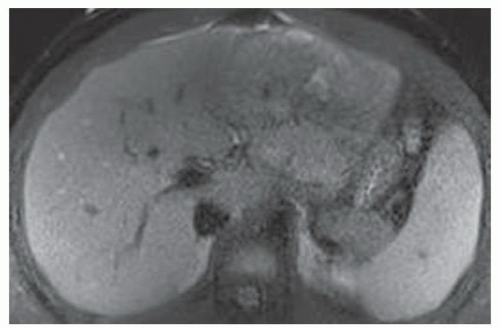
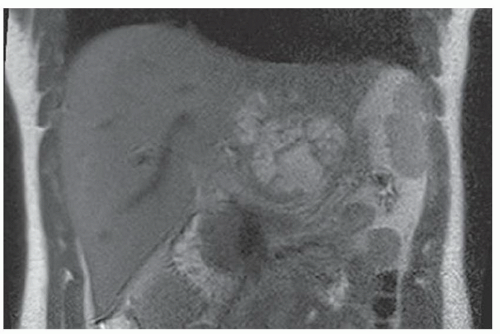
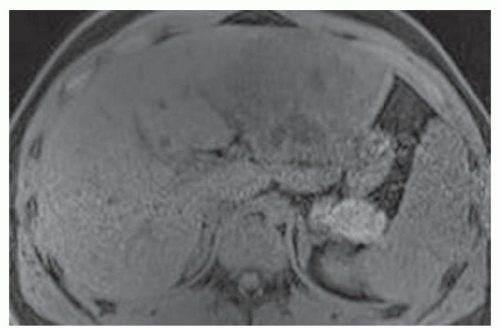
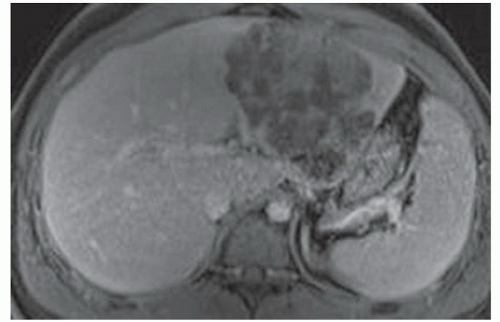
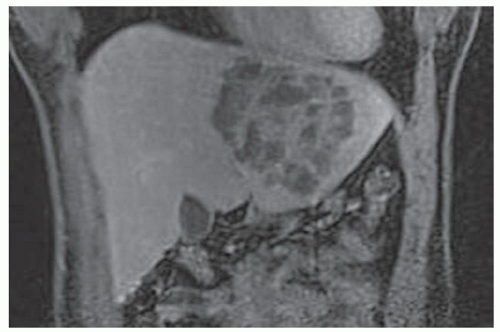
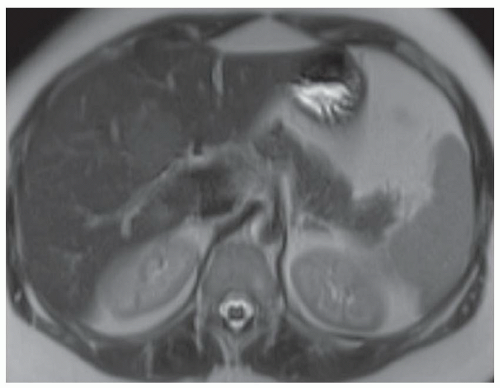
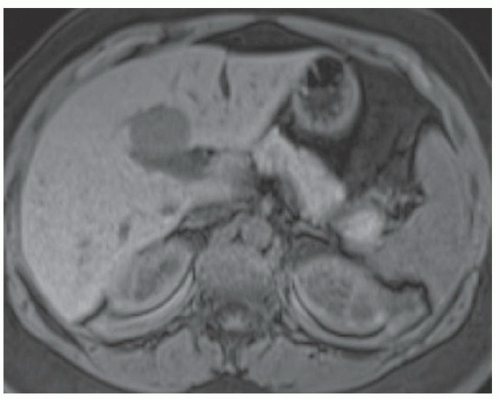
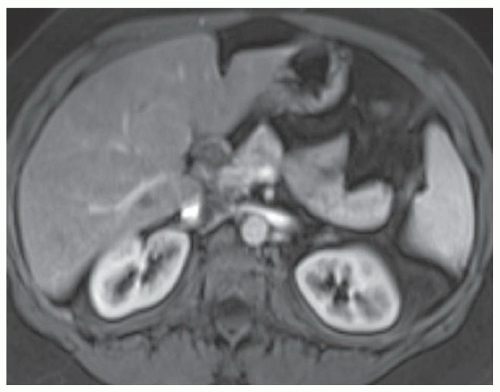
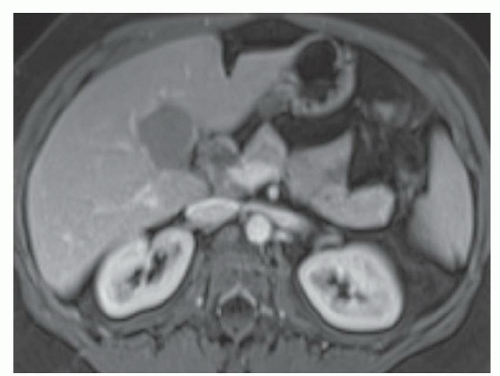
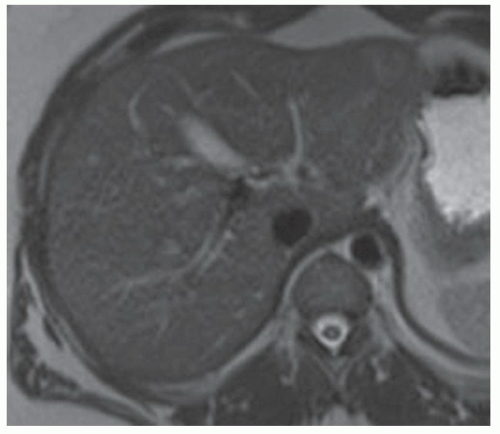
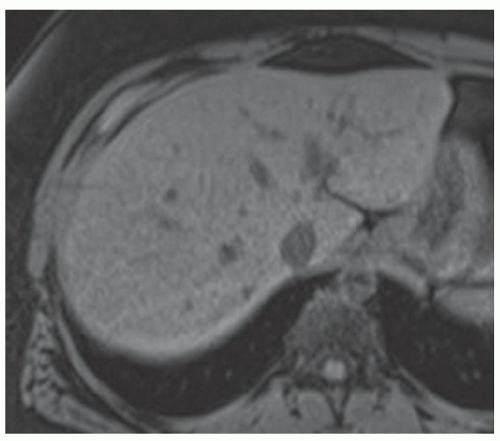
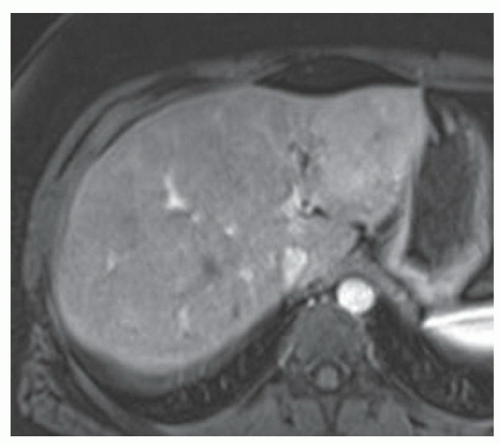
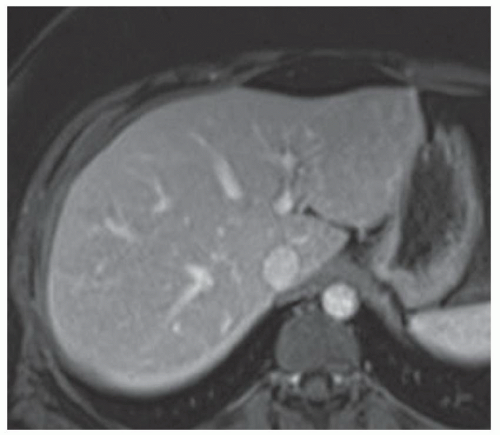
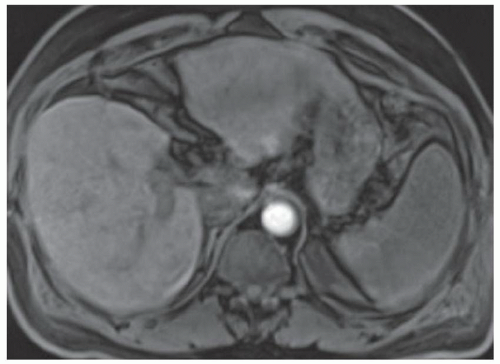
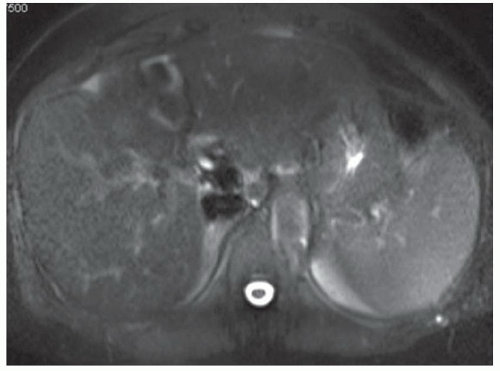
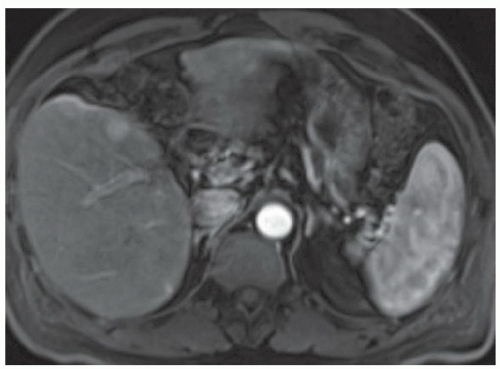
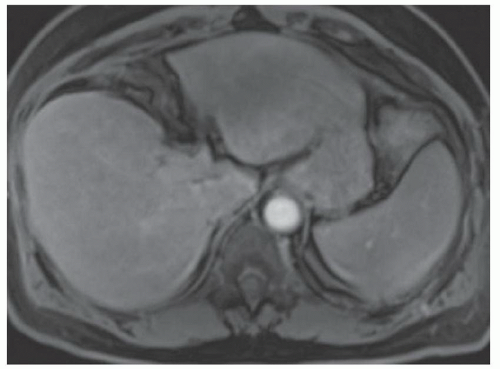
FINDINGS T2 MR (A) demonstrates innumerable subcenti-meter T2 bright structures in the liver parenchyma. These structures demonstrate corresponding T1 dark signal (B) and do not enhance at postcontrast imaging (C). Coronal MRCP slab image (D) also demonstrates these innumerable subcentimeter T2 bright cystic structures diffusely involving the right and left hepatic lobes.
DIFFERENTIAL DIAGNOSIS Liver cysts, metastases, micro-abscesses, von Meyenburg complexes.
DIAGNOSIS Cystic bile duct hamartomas (von Meyenburg complexes).
DISCUSSION The well-circumscribed morphology and homogeneous fluid bright signal (as bright as the CSF) in the above structures make liver cysts or von Meyenburg complexes the most likely etiology. Although metastases are often bright at T2 MR and areas of necrosis may appear fluid bright, the entire metastasis is usually not homogeneously fluid bright as in the above case. Similarly, the liquefied
portion of an abscess may demonstrate fluid bright signal but the entire abscess is usually not homogeneously fluid bright.
portion of an abscess may demonstrate fluid bright signal but the entire abscess is usually not homogeneously fluid bright.
True hepatic cysts are thought to originate from ham-artomatous tissue and do not communicate with the biliary system. At histology, true hepatic cysts are lined by biliary type epithelium. Hepatic cysts are usually homogeneously dark at T1 MR, homogeneously bright at T2 MR, and do not enhance. Hepatic cysts may demonstrate intrinsic T1 bright signal if complicated by internal blood products.
There is some controversy as to whether hepatic cysts are all cystic bile duct hamartomas. Cystic bile duct hamartomas, also known as von Meyenburg complexes, are also cystic structures lined by biliary epithelium. It has been postulated that biliary hamartomas result from failure of involution of embryonic bile ducts. von Meyenburg complexes demonstrate homogeneously T1 dark signal, homogeneously T2 bright signal, and do not enhance. In other words, von Meyenburg complexes and cysts are indistinguishable at imaging and some authors believe that these structures are the same pathologic entity.
Question for Further Thought
1. Are cystic bile duct hamartomas (von Meyenburg complexes) thought to be symptomatic or asymptomatic?
View Answer
1. Cystic bile duct hamartomas are thought to be asymptomatic unless complicated by infection or hemorrhage.
Reporting Requirement
1. Describe the presence of innumerable subcentimeter cystic structures in the liver in keeping with innumerable cystic bile duct hamartomas or liver cysts.
What the Treating Physician Needs to Know
1. Tiny cystic bile duct hamartomas such as those above are thought to have no clinical significance.
Answer
1. Cystic bile duct hamartomas are thought to be asymptomatic unless complicated by infection or hemorrhage.
REFERENCES
1. Mortele KJ, Ros PR. Cystic focal liver lesions in the adult: differential CT and MR imaging features. Radiographics 2001; 895-910.
2. Vachha B, Sun MRM, Siewert B, Eisenberg RL. Cystic lesions of the liver. Am J Roentgenol 2011;196:W355-W366.
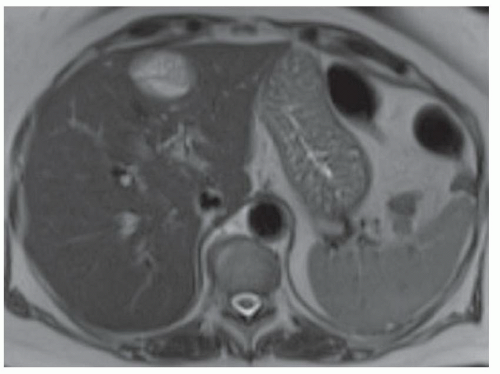
CASE 3.2 CLINICAL HISTORY 45-year-old woman with abdominal pain.
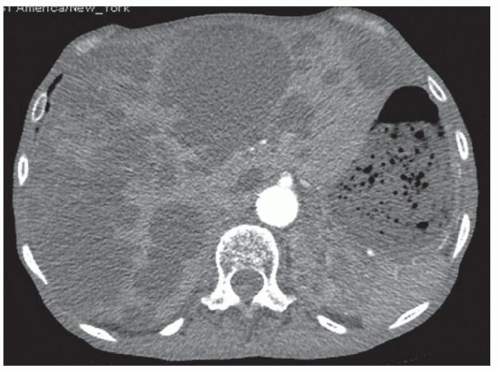
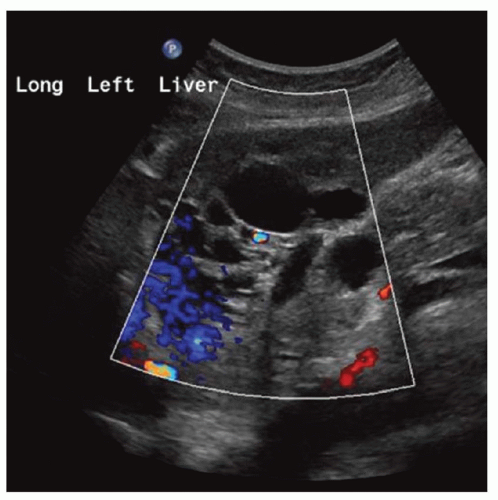
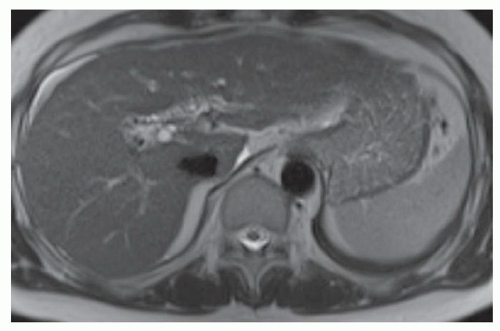
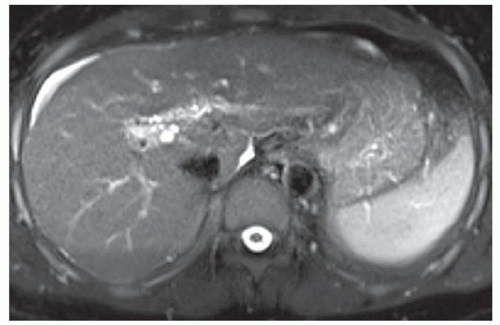
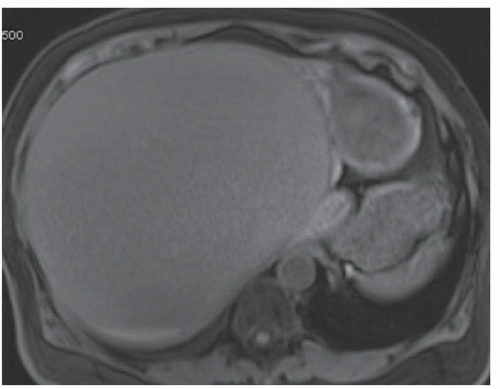
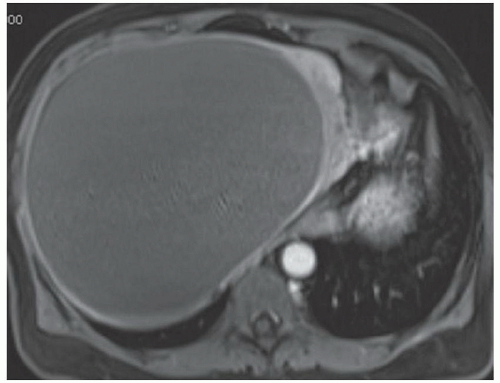
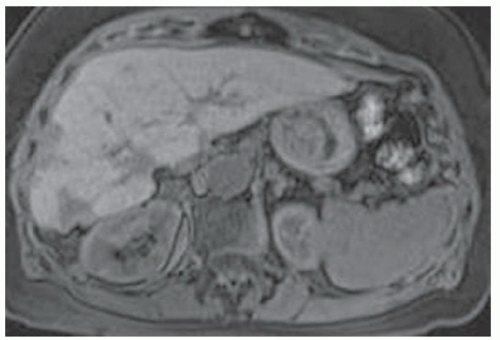
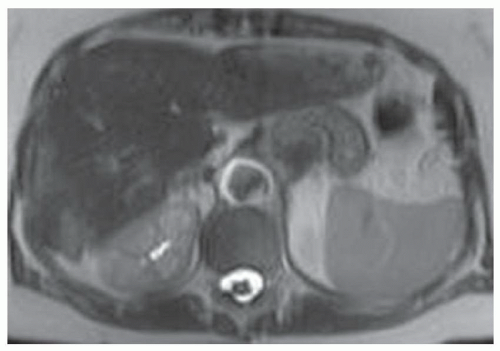
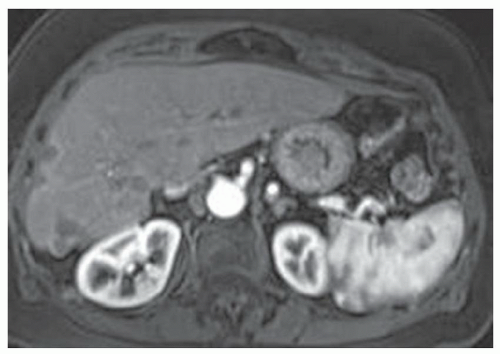
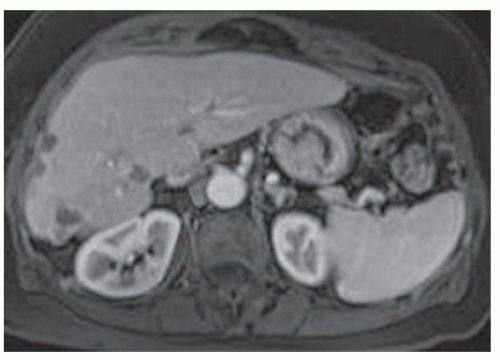
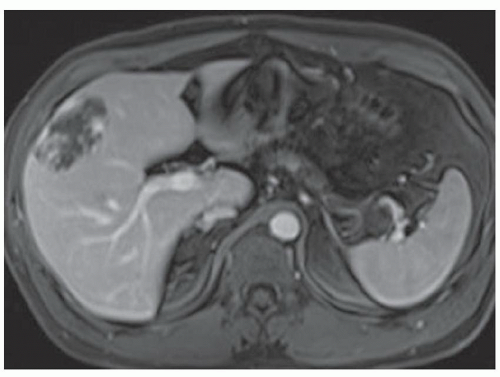
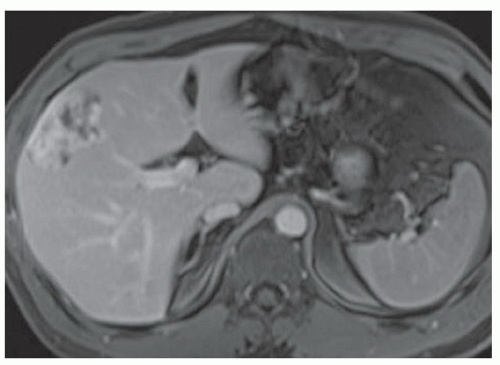
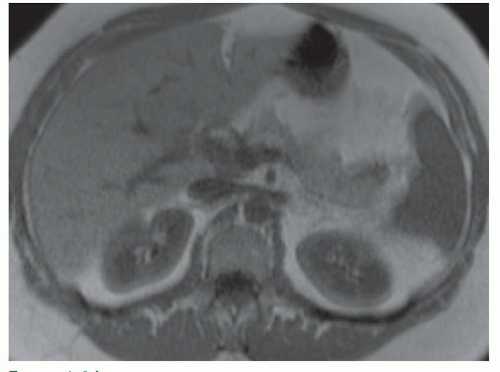
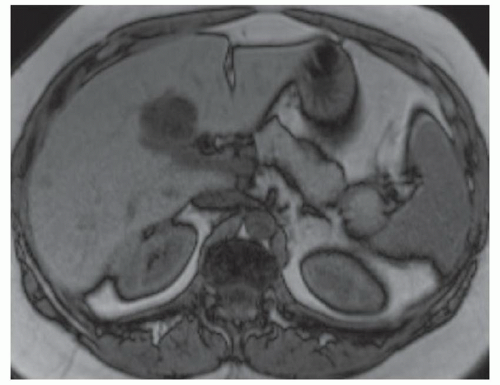
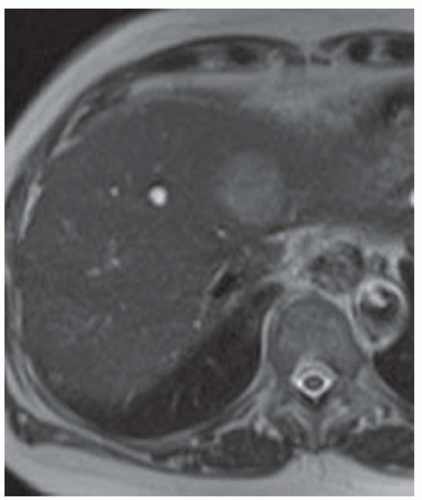
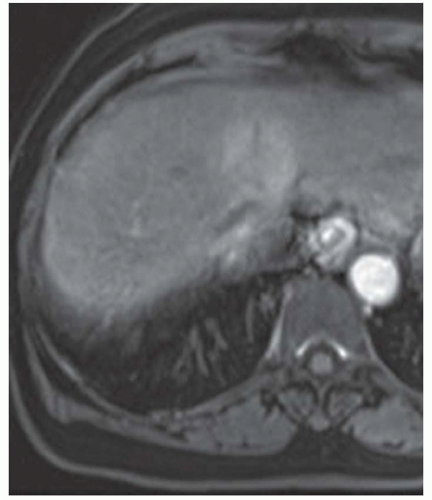
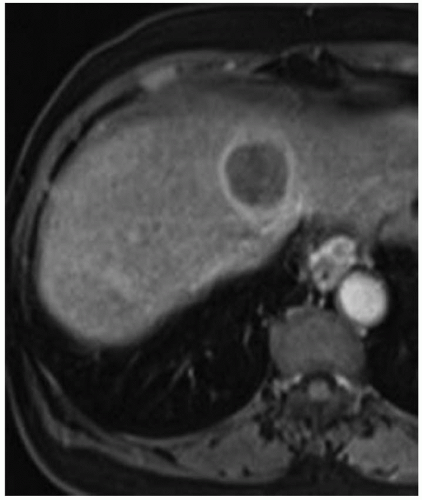
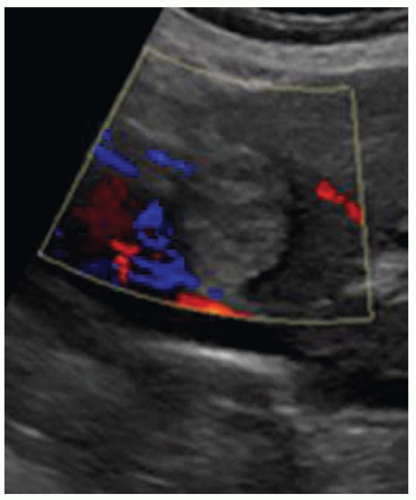
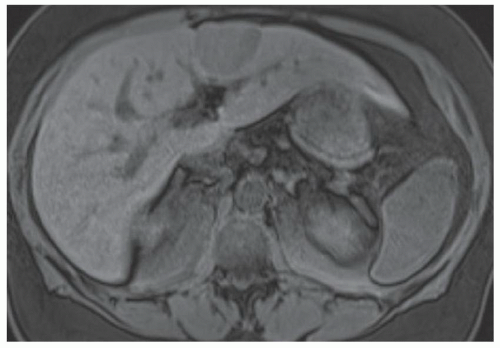
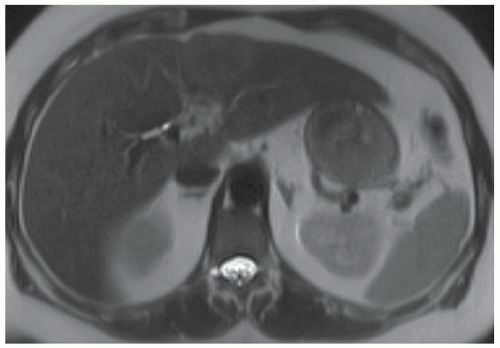
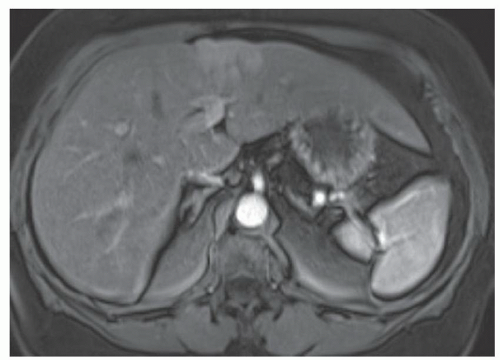
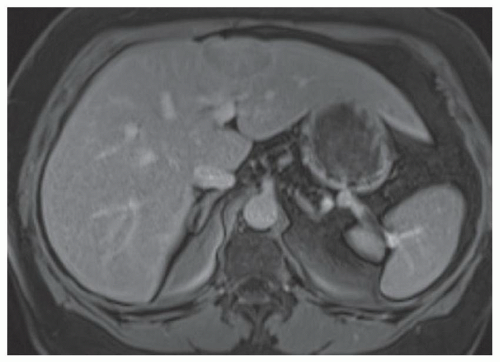
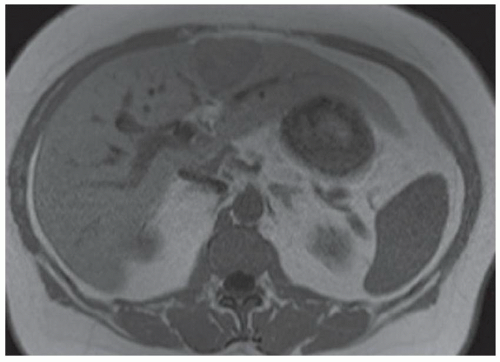
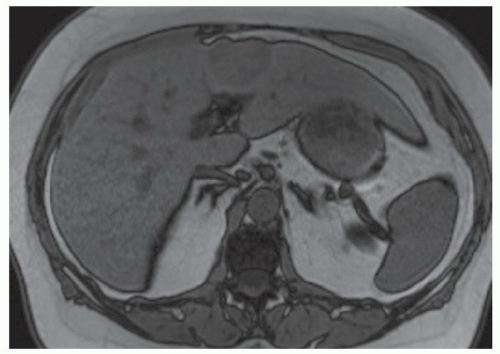
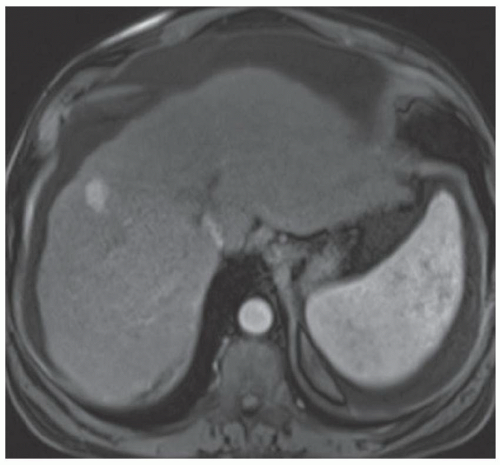
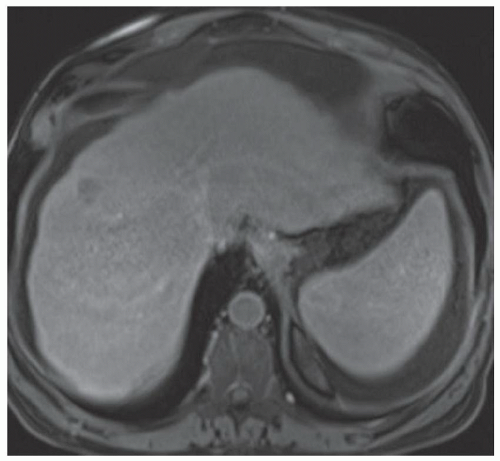
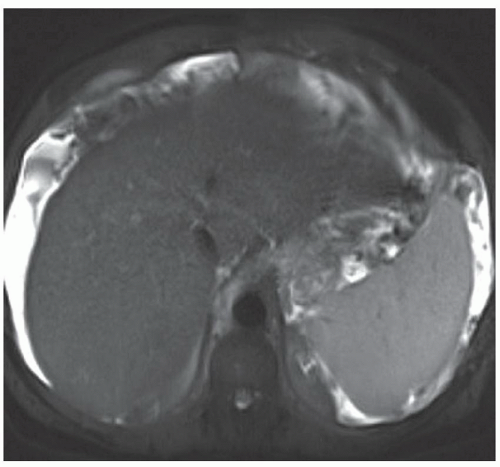
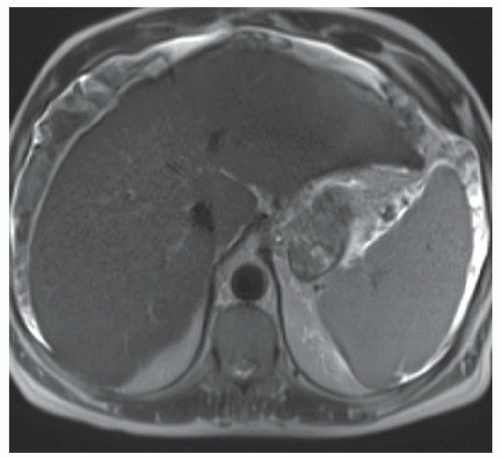
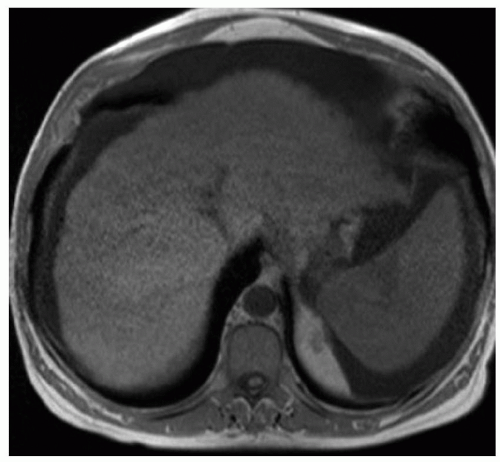
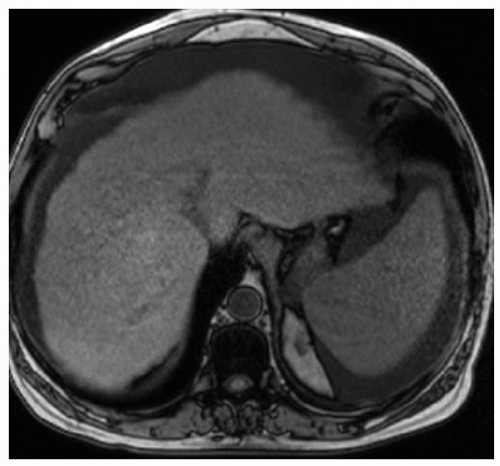
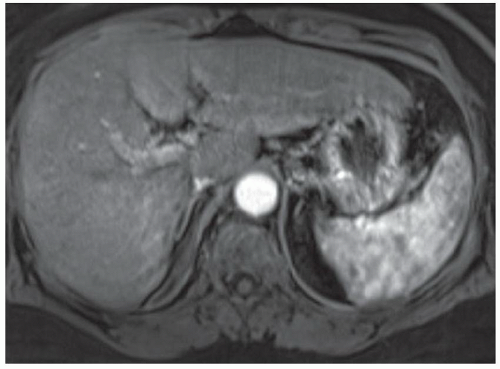
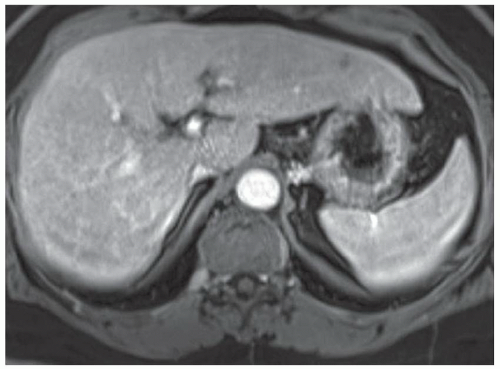
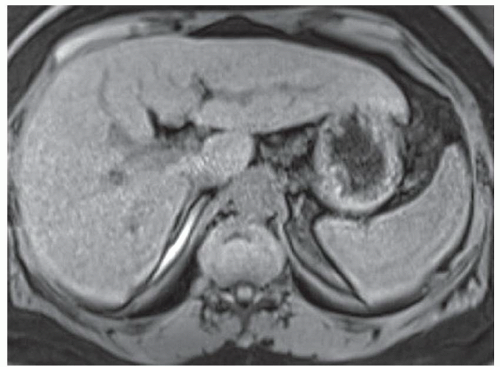
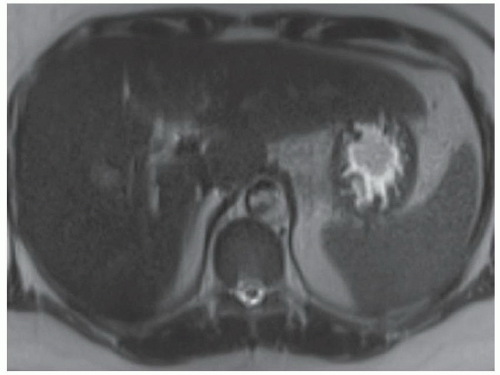
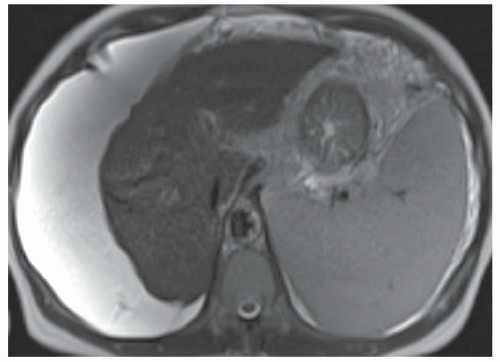
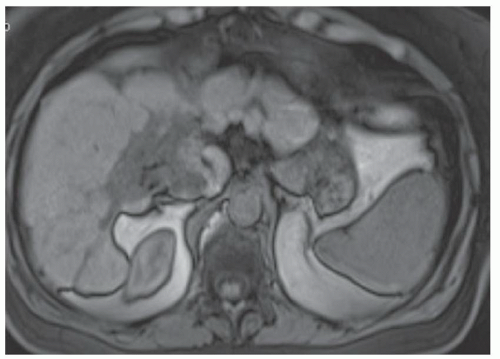
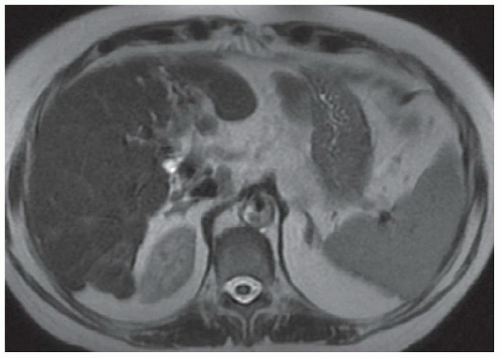
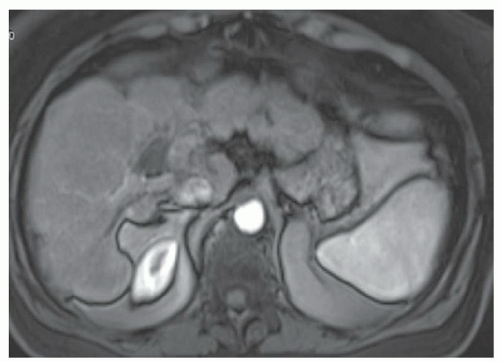
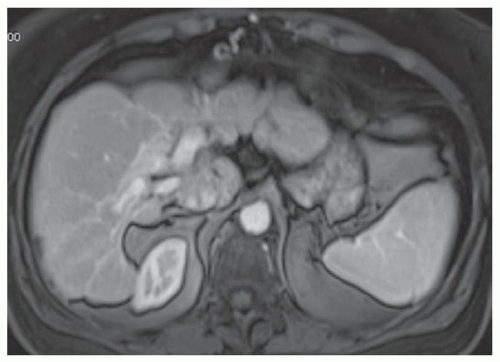
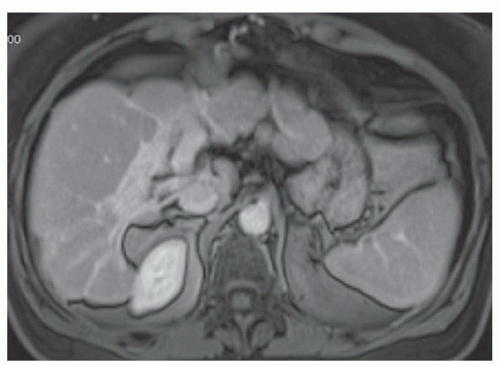
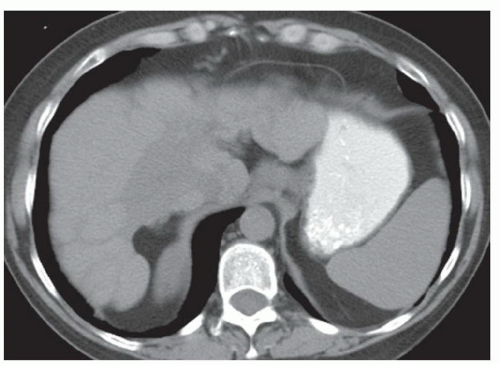
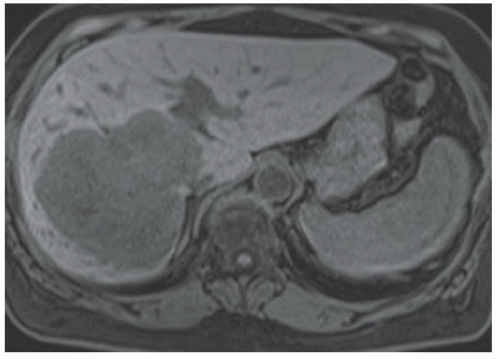
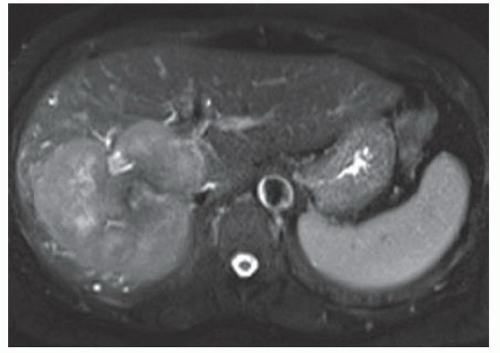
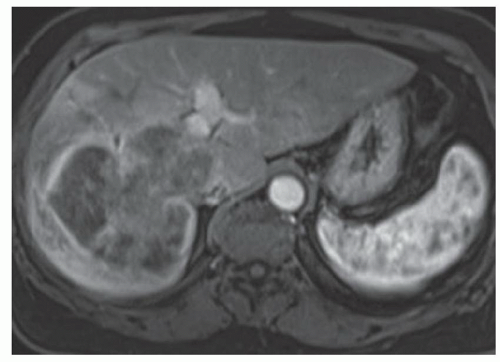
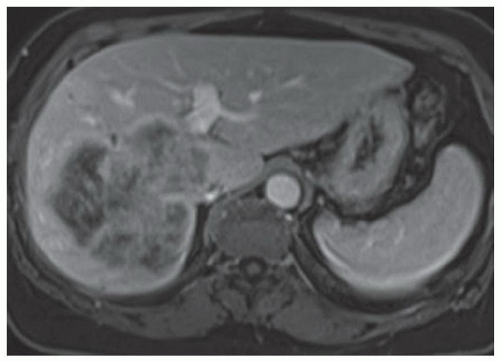
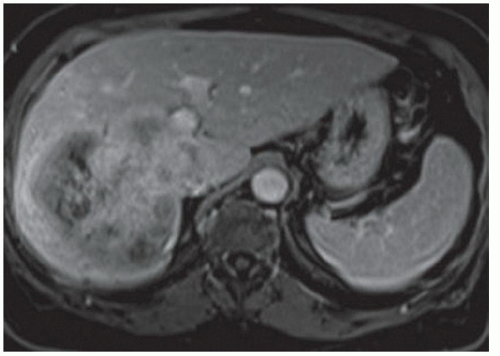
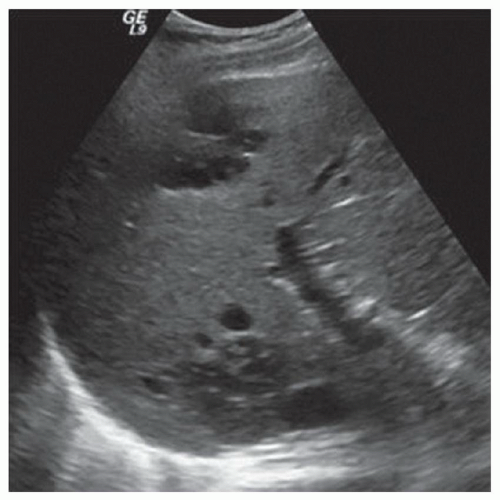
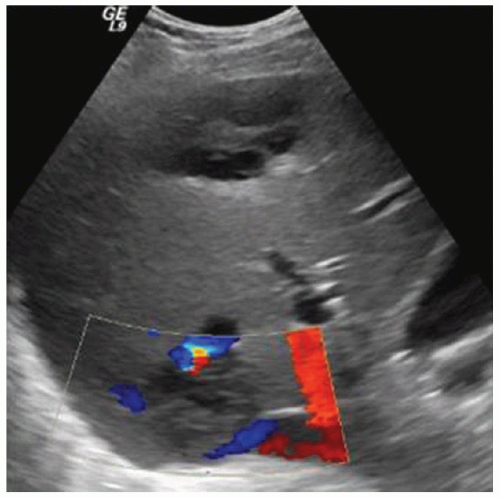
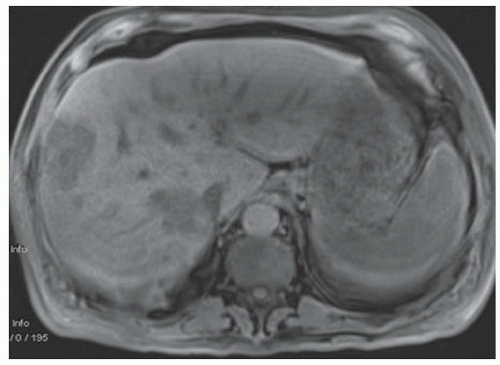
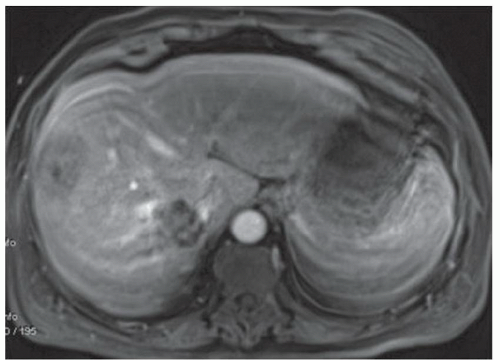
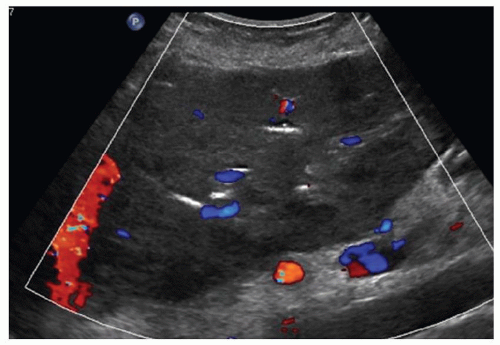
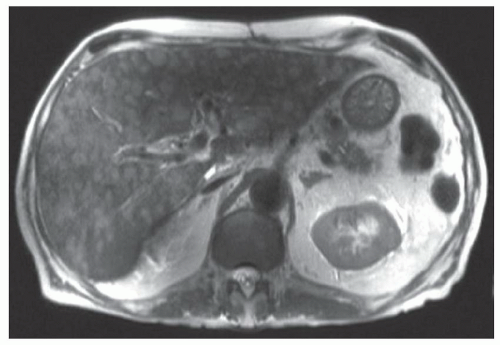
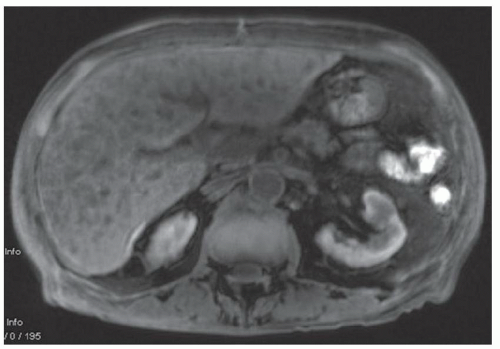
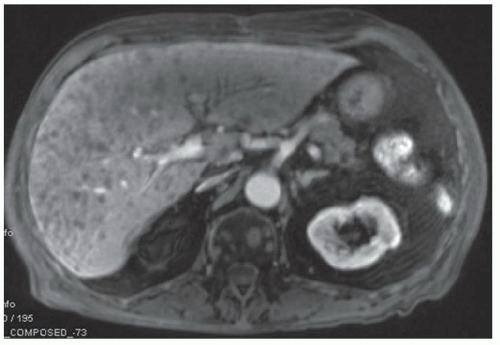
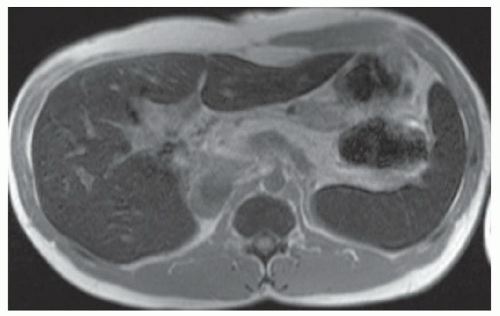
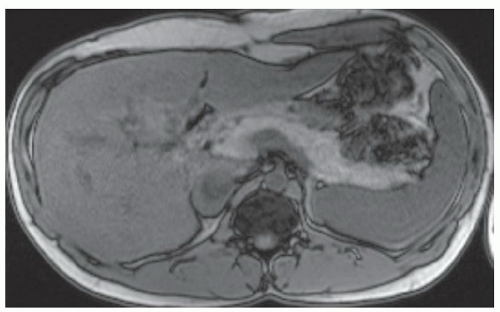
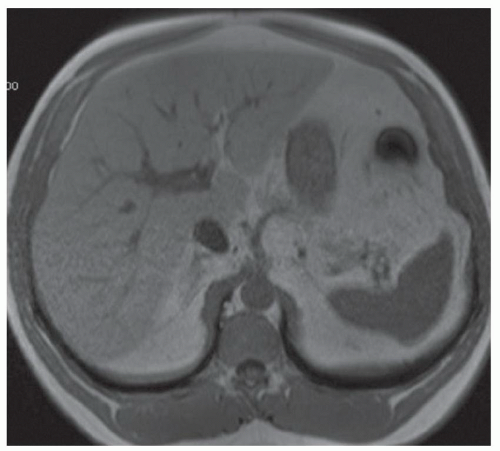
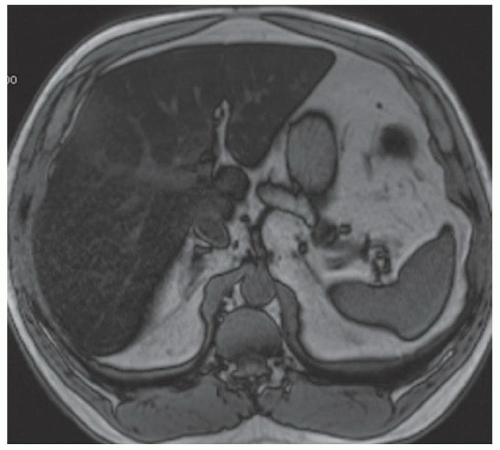
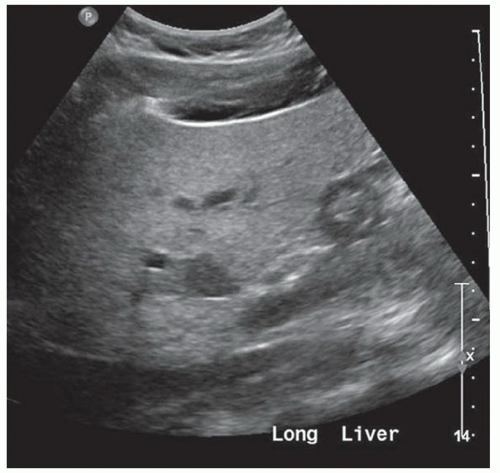
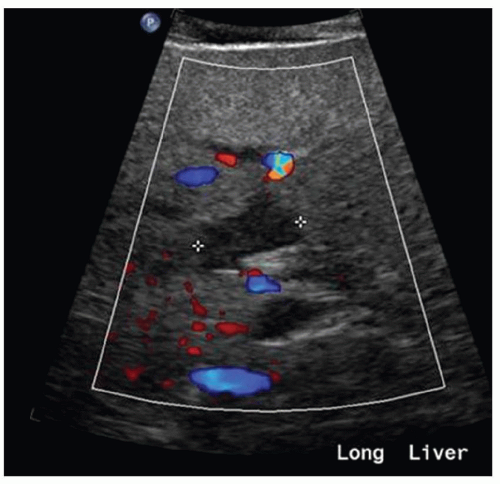
FINDINGS Numerous (>20) well-circumscribed T2 bright (A) and T1 dark (B) structures are present in the liver and replace more than 50% of the hepatic parenchyma. No enhancement was seen in these structures after administration of intravenous contrast material (C). The patient also has numerous renal cysts, best seen in the coronal T2-weighted image (D). Note that some of the renal and liver lesions contain intrinsic T1 bright material reflecting small-volume blood products or proteinaceous debris (D). Numerous non-enhancing structures are also present at contrast-enhanced CT (E). Ultrasound (F) demonstrates anechoic structures with increased through transmission and no internal blood flow.
DIFFERENTIAL DIAGNOSIS Abscesses, cystic liver metastases, polycystic liver disease.
DIAGNOSIS Polycystic liver disease associated with autosomal-dominant polycystic kidney disease (ADPCKD).
DISCUSSION Polycystic liver disease most commonly occurs in association with ADPCKD but may also occur in isolation due to a separate genetic mutation that results in autosomal-dominant polycystic liver disease. Polycystic liver disease occurs in approximately 30% to 70% of patients with ADPCKD due to mutations on chromosomes 4 and 16. The genetic mutations that result in isolated autosomal-dominant polycystic liver disease have been isolated to chromosomes 6 and 19.
The cysts in polycystic liver disease are bile duct hamartomas that are lined with biliary epithelium but have no connection to the biliary system. Over time, the epithelial lining secretes fluid and the cysts progressively enlarge.
Most patients with polycystic liver disease are asymptomatic, and liver function tests are usually normal. However, a minority of patients will develop massive hepatomegaly and secondary symptoms of abdominal pain, distention, and early satiety or dyspnea due to mass effect from the massively enlarged liver. Rarely, liver cysts may hemorrhage or become infected.
At MR, the cysts associated with polycystic liver disease appear as well-circumscribed T2 bright structures. T1 signal will vary depending on cyst content. Cysts are usually dark on T1-weighted imaging but may demonstrate intrinsic T1 bright signal if they contain blood products or proteinaceous debris. If a cyst becomes infected, it may demonstrate perilesional edema and rim and perilesional enhancement. At CT, polycystic liver disease appears as multiple well-circumscribed and nonenhancing cysts. At ultrasound, cysts associated with polycystic liver disease appear as anechoic (black) structures with increased through transmission and no internal blood flow.
Abscesses are not as homogeneous in signal intensity, may demonstrate adjacent edema, and often demonstrate abnormal enhancement such as internal septations or peripheral enhancement. Cystic liver metastases usually can be distinguished from polycystic liver disease as cystic metastases usually have a soft-tissue component and will demonstrate areas of enhancement after administration of intravenous contrast material.
Questions for Further Thought
1. What are treatment options for patients with polycystic liver disease?
View Answer
1. No known medical therapy will halt cyst enlargement. Aspiration of a dominant cyst may provide brief partial symptomatic relief, but fluid within the cyst will reaccumulate. Surgical unroofing (also known as fenestration) of a cyst may be performed whereby the operating surgeon will attempt to remove as much of the cyst wall as possible. In rare cases, liver transplant may be performed for patients with severe refractory symptoms.
2. How many cysts are necessary for a diagnosis of polycystic liver disease?
View Answer
2. Different sources suggest different definitions of the number of cysts necessary for a diagnosis of polycystic liver disease. In general, more than 20 cysts should be present before a diagnosis of polycystic liver disease is invoked. Patients with fewer scattered liver cysts that occupy a small percentage of total hepatic parenchyma should not be diagnosed with polycystic liver disease based on imaging.
Reporting Requirements
1. Describe the size and distribution of cysts with reference measurements of some of the larger cysts.
2. Report if the enlarged liver appears to be causing mass effect on adjacent structures such as bowel and diaphragm.
3. Report if cysts contain internal blood products or evidence of infection.
What the Treating Physician Needs to Know
1. If the patient has massive hepatomegaly with mass effect on adjacent structures.
2. If a cyst appears to contain new hemorrhage or be infected as either scenario could be a cause of patient symptoms.
Answers
1. No known medical therapy will halt cyst enlargement. Aspiration of a dominant cyst may provide brief partial symptomatic relief, but fluid within the cyst will reaccumulate. Surgical unroofing (also known as fenestration) of a cyst may be performed whereby the operating surgeon will attempt to remove as much of the cyst wall as possible. In rare cases, liver transplant may be performed for patients with severe refractory symptoms.
2. Different sources suggest different definitions of the number of cysts necessary for a diagnosis of polycystic liver disease. In general, more than 20 cysts should be present before a diagnosis of polycystic liver disease is invoked. Patients with fewer scattered liver cysts that occupy a small percentage of total hepatic parenchyma should not be diagnosed with polycystic liver disease based on imaging.
REFERENCES
1. Morgan DE, Lockhart ME, Canon CL, Holcombe MP, Bynon JS. Polycystic liver disease: multimodality imaging for complications and transplant evaluation. Radiographics 2006;26:1655-1668.
2. Brancatelli G, Federle MP, Vilgrain V, Vullierme M-P, Marin D, Lagalla R. Fibropolycystic liver disease: CT and MR imaging findings. Radiographics 2005;25:659-670.
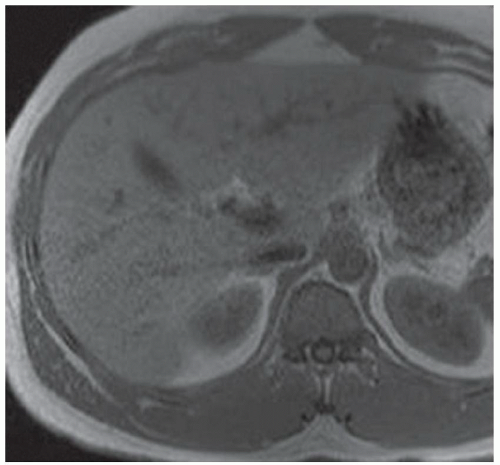
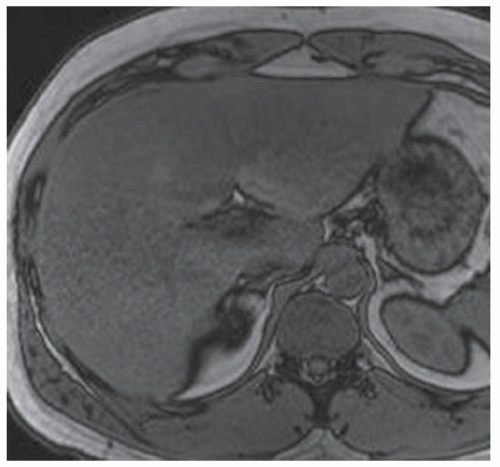
CASE 3.3 CLINICAL HISTORY 49-year-old man with hepatitis C undergoing MR screening for hepatocellular cancer.
FINDINGS T2-weighted MR (A) and T2-weighted MR with fat saturation (B) demonstrate numerous 2- to 4-mm cystic structures along the central intrahepatic biliary system. This finding is confirmed in the True FISP image (C) as well as at magnetic resonance cholangiopancreatography (MRCP) (D).
DIFFERENTIAL DIAGNOSIS Peribiliary cysts.
DIAGNOSIS Peribiliary cysts.
DISCUSSION Peribiliary cysts are thought to form when periductal glands become obstructed as a result of inflammation. Given the increased frequency of peribiliary cysts in patients with severe liver disease, portal hypertension, and portal vein occlusion, it has been hypothesized that elevated portal vein pressures may trigger the inciting inflammatory process. These cysts are usually asymptomatic though cases where large peribiliary cysts resulted in biliary obstruction have been described.
Peribiliary cysts can be detected as small cystic structures along the central intrahepatic biliary system at ultrasound, CT, or MR. At ultrasound, these structures may be difficult to identify but when visible appear hypo- to anechoic with increased through transmission and no internal blood flow. At CT and MR, these structures appear as nonenhancing fluid attenuation and fluid signal structures. At MR, these structures are most readily seen at T2-weighted imaging.
In one series of 12 patients, peribiliary cysts ranged in size from 0.3 to 2.5 cm. The most common morphology of peribiliary cysts is multiple adjacent round cysts with the appearance of beads on a string. When small, these peribiliary cysts may mimic periportal edema. Less commonly,
peribiliary cysts appear as dilated tubular structures that are difficult to distinguish from dilated bile ducts.
peribiliary cysts appear as dilated tubular structures that are difficult to distinguish from dilated bile ducts.
Questions for Further Thought
1. What is the clinical significance of peribiliary cysts?
2. Do peribiliary cysts require any specific treatment?
View Answer
2. In most cases, no. If peribiliary cysts are resulting in biliary ductal dilatation, the ductal dilatation will be treated.
Reporting Requirements
1. Small peribiliary cysts likely warrant only a brief mention in the body of the report.
2. Large peribiliary cysts resulting in biliary ductal dilatation should be discussed in the impression of the radiology report.
What the Treating Physician Needs to Know
1. Peribiliary cysts usually warrant no specific treatment unless they are causing biliary ductal dilatation.
Answers
1. These structures are usually asymptomatic.
2. In most cases, no. If peribiliary cysts are resulting in biliary ductal dilatation, the ductal dilatation will be treated.
REFERENCES
1. Baron RL, Campbell WL, Dodd III GD. Peribiliary cysts associated with severe liver disease: imaging-pathologic correlation. Am J Roentgenol 1994;162:631-636.
2. Seguchi T, Akiyama Y, Itoh H, et al. Multiple hepatic peribiliary cysts with cirrhosis. J Gastroenterol 2004;39:384-390.
CASE 3.4 CLINICAL HISTORY 68-year-old woman with right upper quadrant pain and shortness of breath.
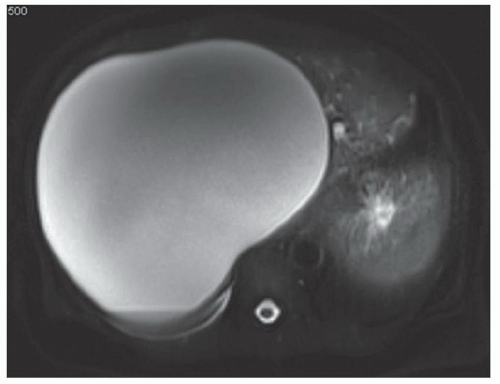
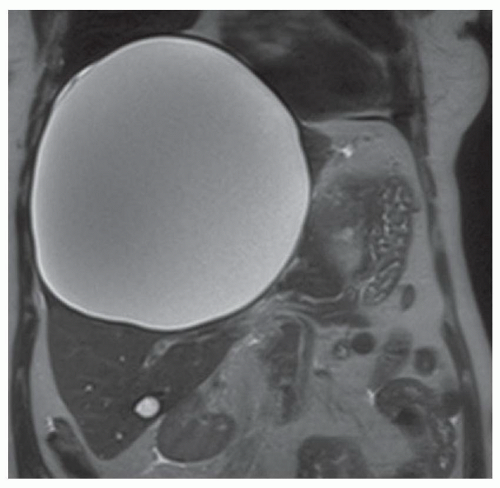
FINDINGS Axial (A) and coronal (B) T2-weighted MR images demonstrate a 19-cm T2 bright structure in the right hepatic lobe. The lesion demonstrates low signal at T1-weighted MR (C) and does not enhance after administration of intravenous contrast material (D). A small amount of layering T2 dark and T1 bright debris is present (A, C). Note the additional similar appearing but smaller lesion in the inferior right hepatic lobe (C).
DIFFERENTIAL DIAGNOSIS Abscess, biliary cystadenoma, hepatic cyst, hydatid cyst.
DIAGNOSIS Hepatic cyst.
DISCUSSION The above lesion is a unilocular cystic structure with a small amount of internal debris and no enhancing component. Abscesses are usually not as well defined and homogeneous as they often demonstrate enhancing septations and adjacent edema. Biliary cystadenomas are multilocular cystic masses. Hydatid cysts usually contain internal daughter cysts and may demonstrate wall calcification at CT.
The etiology of liver cysts is unknown. It has been postulated that these cystic structures are hamartomas that do not develop a normal connection to the biliary tree. These cysts are lined by epithelium that can secrete fluid and result in cyst enlargement.
Hepatic cysts are usually asymptomatic and no treatment is required. When large, these cysts may produce symptoms due to mass effect such as early satiety (if mass effect on the stomach) and shortness of breath (if diaphragm elevation).
Questions for Further Thought
1. Should image-guided cyst aspiration be performed for symptomatic relief?
View Answer
1. Aspiration of hepatic cysts is usually not recommended as these cysts will rapidly reaccumulate fluid.
2. What surgical procedure may be performed to treat a symptomatic liver cyst?
View Answer
2. Cyst unroofing is the preferred treatment for symptomatic liver cysts. The procedure may be performed laparoscopically or via an open approach. The aim of the procedure is to remove as much of the cyst wall as is possible by removing tissue at the cyst-liver interface.
Reporting Requirements
1. Describe the approximate number of cysts, location, and size of the largest cysts.
2. Describe evidence of mass effect such as mass effect on the stomach or diaphragm elevation.
What the Treating Physician Needs to Know
1. It is critically important to try to distinguish between a simple cyst and a hydatid cyst at imaging. The unroofing procedure performed for a simple cyst would be contraindicated for a hydatid cyst as spilling of hydatid cyst contents into the peritoneal cavity could result in anaphylactic shock. In patients with equivocal imaging, this uncertainty should be conveyed in the imaging report so that the operative surgeon can perform appropriate testing (e.g., blood testing for parasites) to rule out hydatid cyst prior to unroofing.
Answers
1. Aspiration of hepatic cysts is usually not recommended as these cysts will rapidly reaccumulate fluid.
2. Cyst unroofing is the preferred treatment for symptomatic liver cysts. The procedure may be performed laparoscopically or via an open approach. The aim of the procedure is to remove as much of the cyst wall as is possible by removing tissue at the cyst-liver interface.
REFERENCES
1. Mortele KJ, Ros PR. Cystic focal liver lesions in the adult: differential CT and MR imaging features. Radiographics 2001;21:895-910.
2. Loehe F, Globke B, Marnoto R, et al. Long-term results after surgical treatment of nonparasitic hepatic cysts. Am J Surg 2010;200:23-31.
CASE 3.5 CLINICAL HISTORY 21-year-old man with 3-day history of abdominal pain, fever, nausea, and vomiting.
FINDINGS Axial T2-weighted MR without (A) and with (B) fat saturation and coronal T2-weighted MR without fat saturation (C) demonstrate a 9-cm structure in the left hepatic lobe containing rounded areas of T2 bright signal abnormality with linear areas of internal T1 and T2 dark signal and no more than minimal adjacent inflammatory changes. T1-weighted MR (D) demonstrates the mass to be of lower signal than the adjacent hepatic parenchyma. Axial (E) and coronal (F) postcontrast MR demonstrate numerous rounded nonenhancing areas of fluid interspersed between irregular areas of enhancement and debris (contrast agent: Gd-BOPTA [MultiHance; Bracco Diagnostics, Milan, Italy]).
DIFFERENTIAL DIAGNOSIS Abscess, biliary cystadenocarcinoma, intrahepatic cholangiocarcinoma.
DIAGNOSIS Abscess.
DISCUSSION Liver abscesses may result from a variety of conditions including gastrointestinal infection with resultant portal vein seeding or systemic sepsis. Patients who have undergone a bilioenteric anastomosis also are at increased risk for liver abscess due to cholangitis. Clinical presentation is highly variable with some patients presenting with fever and right-sided abdominal pain and other patients presenting with more vague abdominal pain.
At CT, ultrasound, and MR, liver abscesses have a varied appearance. Multiple abscesses may be visible scattered throughout the entire liver or a more focal area of infection may be visible. Individual abscesses may appear as unilocular or multilocular.
At CT, abscesses appear as multiple randomly distributed lesions or as clustered areas of hypoenhancing tissue or fluid. These structures have more ill-defined margins than simple liver cysts. At CT, abscesses may demonstrate a thick rim and enhancing internal septations. At ultrasound, small abscesses may appear as hypoechoic masses that can be difficult to distinguish from solid masses. As abscesses enlarge, mobile debris may be visible at ultrasound which is a clue to the diagnosis.
At MR, abscesses usually demonstrate T2 bright signal reflecting their fluid component. T2 signal may vary from mildly bright to fluid bright depending on the nature of the fluid component. Additionally, T2-weighted images with fat saturation may demonstrate bright signal, indicating edema within the adjacent liver or perihepatic fat. T1 signal intensity varies depending on the amount of fluid versus cellular debris present. Postcontrast images often demonstrate enhancing internal septations and rim-enhancing tissue.
Questions for Further Thought
1. Would this lesion be amenable to percutaneous drainage catheter placement?
View Answer
1. Aspirating fluid from this abscess for culture is reasonable. Once a causative organism and antibiotic sensitivities are definitively known, an optimized antibiotic regimen can be prescribed. It is not unreasonable to place a percutaneous drainage catheter in the abscess to attempt to remove as much fluid as possible. A percutaneous drainage catheter was placed in this case, but given the multiloculated nature of the abscess only a small amount of fluid was drained. At follow-up imaging several days later, the abscess had not decreased in size. The patient therefore underwent left hepatectomy with gross pathology revealing an 11-cm abscess.
2. What percentage of patients exhibit signs and symptoms of sepsis immediately following percutaneous drainage of a liver abscess?
View Answer
2. Up to 26% of patients may develop sepsis within 15 to 30 minutes of placement of a percutaneous drainage catheter in a liver abscess. Signs of sepsis include fever, hypotension, rigors, and hypoxemia. Antibiotics should be administered prior to percutaneous drainage of a liver abscess. Additionally, excessive manipulation of the abscess should be avoided.
Reporting Requirements
1. Call the ordering physician to alert him/her to this unexpected finding.
2. Describe the size and location of the abnormality.
What the Treating Physician Needs to Know
1. Given lesion appearance and patient history, this finding is most worrisome for a large abscess.
2. Consideration could be given to imaging the pelvis (to evaluate for diverticulitis or appendicitis) and dental examination to evaluate for potential source of infection.
Answers
1. Aspirating fluid from this abscess for culture is reasonable. Once a causative organism and antibiotic sensitivities are definitively known, an optimized antibiotic regimen can be prescribed. It is not unreasonable to place a percutaneous drainage catheter in the abscess to attempt to remove as much fluid as possible. A percutaneous drainage catheter was placed in this case, but given the multiloculated nature of the abscess only a small amount of fluid was drained. At follow-up imaging several days later, the abscess had not decreased in size. The patient therefore underwent left hepatectomy with gross pathology revealing an 11-cm abscess.
2. Up to 26% of patients may develop sepsis within 15 to 30 minutes of placement of a percutaneous drainage catheter in a liver abscess. Signs of sepsis include fever, hypotension, rigors, and hypoxemia. Antibiotics should be administered prior to percutaneous drainage of a liver abscess. Additionally, excessive manipulation of the abscess should be avoided.
REFERENCES
1. Thomas J, Turner SR, Nelson RC, Paulson EK. Postprocedure sepsis in imaging-guided percutaneous hepatic abscess drainage: how often does it occur? Am J Roentgenol 2006;186: 1419-1422.
2. Mortele KJ, Segatto E, Ros PR. The infected liver: radiologicpathologic correlation. Radiographics 2004;24:937-955.
CASE 3.6 CLINICAL HISTORY 63-year-old woman; MR performed to characterize liver lesions identified on a CT scan performed in the emergency department to evaluate abdominal pain.
FINDINGS Precontrast T1-weighted MR (A) demonstrates several ill-defined, low-signal lesions in the right hepatic lobe that are bright on T2-weighted imaging (B). Note also associated capsular retraction about the lesions. Arterial phase imaging with Gd-BOPTA (MultiHance; Bracco Diagnostics, Milan, Italy) demonstrates peripheral rim and perilesional enhancement (C) with subtle increase in enhancement of the most posterior lesion in the venous phase images (D).
DIFFERENTIAL DIAGNOSIS Hepatic epithelioid hemangio-endothelioma, metastatic disease, multifocal hepatocellular cancer, “pseudocirrhosis” reflecting treated metastatic disease.
DIAGNOSIS Hepatic epithelioid hemangioendothelioma.
DISCUSSION Hepatic epithelioid hemangioendothelioma is a rare tumor of vascular origin. These tumors are often (>80%) multifocal and coalesce over time. Tumors may be peripheral in location with overlying capsular retraction. Lesions demonstrate peripheral rim enhancement and perilesional enhancement. Pulmonary nodules are the most common location for extrahepatic disease followed by nodal, omental, and mesenteric masses.
When imaging features are suggestive of epithelioid hemangioendothelioma, biopsy is needed to confirm the diagnosis. These tumors are often initially misdiagnosed at histology and immunostaining for endothelial markers is required to make the diagnosis. Therefore, mentioning epithelioid hemangioendothelioma in the imaging differential diagnosis can aid the pathologist in making the correct histologic diagnosis. Patients may present with vague symptoms of abdominal discomfort, weight loss, or be asymptomatic. If untreated, lesions usually enlarge and coalesce resulting in liver failure.
The capsular retraction associated with individual liver lesions seen in the present case can also be seen in the setting of treated metastatic disease. This “pseudocirrhosis” appearance has been described, for example, with treated breast cancer metastases. The patient in this case did not have a history of known cancer. The lesions in this case are difficult to distinguish from metastatic disease in general, though the overlying capsular retraction is somewhat atypical for untreated metastatic disease.
Unlike the present case, hepatocellular carcinomas are classically hyperenhancing (not rim enhancing) in the arterial phase and show washout often with a capsule or pseudo-capsule in delayed images. The nodular morphology of the liver in this case is due to focal areas of capsular retraction overlying individual liver lesions. Uninvolved portions of the liver (e.g., left hepatic lobe in the above images) have a normal morphology.
Question for Further Thought
1. Is hepatic epithelioid hemangioendothelioma a benign or malignant tumor?
View Answer
1. Hepatic epithelioid hemangioendothelioma is a tumor of intermediate malignant potential.
Reporting Responsibilities
1. Describe the size, number, and location of the tumor(s).
2. Suggest that tissue sampling may be required for diagnosis.
What the Treating Physician Needs to Know
1. That epithelioid hemangioendothelioma is a differential diagnostic consideration so that immunostains for endothelial markers can be performed on biopsy samples.
2. Is there metastatic disease?
Answer
1. Hepatic epithelioid hemangioendothelioma is a tumor of intermediate malignant potential.
REFERENCES
1. Lyburn ID, Torreggiani WC, Harris AC, et al. Hepatic epithelioid hemangioendothelioma: sonographic, CT, and MR imaging appearances. Am J Roentgenol 2003;180:1359-1364.
2. Earnest IV F, Johnson CD. Case 96: Hepatic epithelioid hemangioendothelioma. Radiology 2006;240:295-298.
CASE 3.7 CLINICAL HISTORY 38-year-old woman with hyperechoic liver lesion seen at ultrasound.
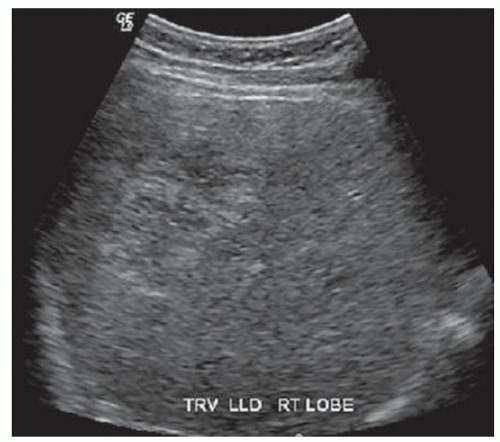
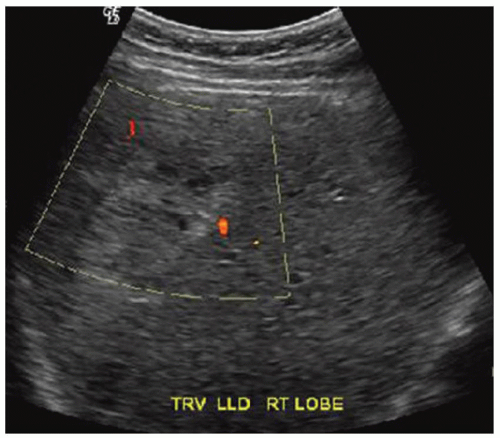
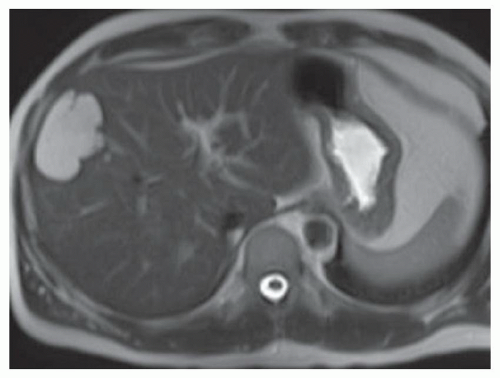
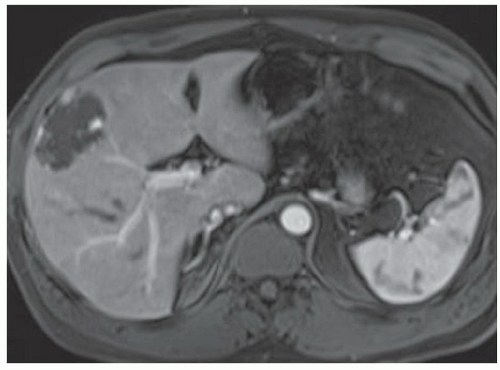
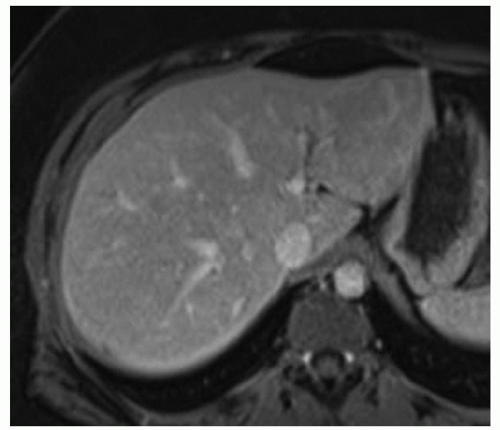
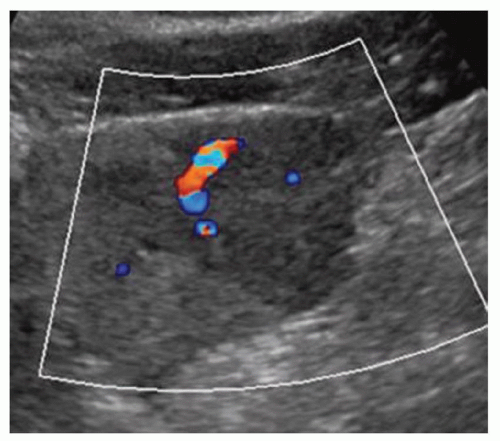
FINDINGS Grayscale ultrasound (A) demonstrates an approximately 4.5-cm hyperechoic lesion in the right hepatic lobe. Color Doppler image (B) demonstrates no definite internal blood flow. Increased through transmission is seen in A and B.
T2-weighted MR demonstrates an ovoid, well-circumscribed homogeneously bright lesion in the right hepatic lobe (C). Arterial phase image (D) acquired 20 seconds after administration of intravenous contrast material demonstrates peripheral, discontinuous nodular enhancement. Venous phase image (E) (acquired 70 seconds after administration of intravenous contrast material) and 3-minute delayed image (F) demonstrate progressive central filling of the lesion (contrast agent: Gd-BOPTA [MultiHance; Bracco Diagnostics, Milan, Italy]).
DIFFERENTIAL DIAGNOSIS Hemangioma, hepatocellular adenoma, hepatocellular carcinoma.
DIAGNOSIS Hemangioma.
Hemangiomas are common tumors occurring in up to 20% of adults and are multiple in up to 40% of patients. These tumors are usually asymptomatic and do not require treatment. In rare cases in which large hemangiomas result in discomfort or are complicated by bleeding, they may be resected.
Histologically, hemangiomas are composed of multiple vascular channels of various sizes and sparse connective tissue. Blood supply is peripheral. Blood then fills in centrally as it makes its way through these dilated vascular channels. Large hemangiomas may contain a fibrous central scar.
At contrast-enhanced cross-sectional imaging (CT or MR), classic hemangiomas demonstrate peripheral discontinuous nodular enhancement with centripetal fill-in. This peripheral nodular enhancement is usually discontinuous and sometimes appears “cloud-like.” The areas of peripheral nodular enhancement are often as bright as the blood pool as in the above case.
At T2-weighted MR, classic hemangiomas are homogeneously bright and may be nearly as bright as the cerebrospinal fluid (CSF). At T2-weighted imaging, hemangiomas and hepatic cysts can appear very similar but can be distinguished in postcontrast imaging based on the classic enhancement pattern of hemangiomas shown above and the absence of hepatic cyst enhancement.
At ultrasound, hemangiomas sometimes appear hyperechoic with increased through transmission and sometimes appear hypoechoic. However, malignant tumors also can appear hyperechoic at ultrasound. CT or MR should be recommended to further characterize indeterminate liver lesions identified at ultrasound. The blood flow in hemangiomas is usually too slow to be detectable at color Doppler or power Doppler ultrasound. If blood flow is detected at ultrasound, the lesion in question is most likely not a hemangioma.
Questions for Further Thought
1. What is Kasabach-Merritt syndrome?
View Answer
1. Kasabach-Merritt syndrome is also known as hemangioma thrombocytopenia syndrome. This syndrome occurs when platelets become sequestered in a vascular tumor such as a hemangioma resulting in a consumptive coagulopathy that can ultimately progress to disseminated intravascular coagulation. Deaths have been reported. Kasabach-Merritt syndrome more commonly occurs in pediatric patients with hemangioendotheliomas but has been described in association with hemangiomas.
2. What is the characteristic enhancement pattern of flash-filling hemangiomas.
View Answer
2. Flash-filling hemangiomas are small lesions that demonstrate marked arterial hyperenhancement in the arterial phase.
Reporting Responsibilities
1. When hemangiomas demonstrate a classic appearance as above, a hemangioma can be confidently diagnosed at imaging.
2. In the setting of extremely large hemangiomas, report if there is mass effect on adjacent organs.
What the Treating Physician Needs to Know
1. Hemangiomas are benign lesions and generally require no further follow-up or treatment.
2. Hemangiomas can be confidently diagnosed at contrast-enhanced cross-sectional imaging and do not require biopsy.
Answers
1. Kasabach-Merritt syndrome is also known as hemangioma thrombocytopenia syndrome. This syndrome occurs when platelets become sequestered in a vascular tumor such as a hemangioma resulting in a consumptive coagulopathy that can ultimately progress to disseminated intravascular coagulation. Deaths have been reported. Kasabach-Merritt syndrome more commonly occurs in pediatric patients with hemangioendotheliomas but has been described in association with hemangiomas.
2. Flash-filling hemangiomas are small lesions that demonstrate marked arterial hyperenhancement in the arterial phase.
REFERENCES
1. Vilanova JC, Barcelo J, Smirniotopolous JG, et al. Hemangioma from head to toe: MR imaging with pathologic correlation. Radiographics 2004;24:367-385.
2. Prasanna PM, Fredericks SE, Winn SS, Christman RA. Giant cavernous hemangioma. Radiographics 2010;30:1139-1144.
CASE 3.8 CLINICAL HISTORY 34-year-old woman with abdominal pain.
FINDINGS An approximately 3.5-cm well-circumscribed lesion is present at the junction of segments 4A and 4B. This lesion demonstrates signal loss at out-of-phase imaging (B) compared with the in-phase imaging (A) indicating the presence of fat. The lesion is faintly bright at T2-weighted MR (C) and is low signal at T1 precontrast MR (D). At dynamic imaging, the lesion hyperenhances in the arterial phase (E) and washes out in the delayed phase (3-minute delayed) images (contrast agent: Gd-BOPTA [MultiHance; Bracco Diagnostics, Milan, Italy]).
DIFFERENTIAL DIAGNOSIS Hemangioma, hepatocellular adenoma, hepatocellular carcinoma.
DIAGNOSIS Hepatocellular adenoma.
DISCUSSION Hepatic adenomas are a benign proliferation of hepatocytes, usually in an otherwise normal liver. Adenomas are relatively rare tumors but occur with increased frequency in women taking oral contraceptive pills, patients with glycogen storage disease, and individuals using anabolic steroids. Adenomas may decrease in size with cessation of oral contraceptive or anabolic steroid use.
At histology, adenomas are characterized by sheets of hepatocytes separated by dilated sinusoids. These dilated, thin-walled sinusoids are perfused by arterial blood pressure from peripheral feeding vessels. Adenomas have minimal supporting connective tissue. As a result of their unique blood supply and lack of supporting connective tissue, adenomas are at increased risk for bleeding. When bleeding does occur, it may spread into the abdominal cavity and be life-threatening as these lesions usually do not have a complete capsule.
Adenoma cells contain large amounts of lipid which is detectable by the loss of signal at out-of-phase imaging. Adenomas are usually mildly hyperintense at T2-weighted imaging though not nearly as bright as hepatic cysts and classic hemangiomas. T1 precontrast signal intensity varies from low signal to high signal with intrinsic T1 bright signal indicating fat or blood products.
At postcontrast imaging, adenomas hyperenhance in the arterial phase and then become isointense to liver or washout at delayed phase imaging. Adenomas are usually sharply marginated, but only a minority of adenomas have a capsule. Calcifications are uncommon but may occur in areas of old hemorrhage or necrosis.
Adenomas may be difficult to distinguish from hepatocellular cancer based on imaging. It is important to note that adenomas typically occur in otherwise normal livers, whereas hepatocellular cancers usually occur in patients with findings of chronic liver disease (e.g., a nodular liver with fibrosis). Adenomas have a low rate of malignant transformation.
Primarily due to their risk of bleeding and also because of a possible low rate of malignant transformation, adenomas are often treated. Treatment options include surgical resection and embolization.
Questions for Further Thought
1. What other liver tumors may contain fat?
View Answer
1. Hepatocellular cancer may contain fat that is detectable at out-of-phase imaging. Hepatic lipomas and angiomyolipomas are relatively rare hepatic tumors that contain gross fat visible as low-attenuation material at CT or detectable based on signal loss seen in MR sequences obtained with fat saturation.
2. Are bile ducts present in adenomas?
View Answer
2. No. Bile ductules are not present in adenomas. By comparison, bile ductules are present in areas of FNH. The absence of bile ductules is a diagnostic criteria used at histology.
Reporting Responsibilities
1. Attempt to distinguish between adenoma and FNH because adenomas are usually treated (e.g., surgery or embolization), whereas FNHs are usually not treated.
2. Attempt to distinguish from hepatocellular cancer. Making this distinction can be difficult, but normal background liver and the appropriate clinical history are supportive of a diagnosis of adenoma.
What the Treating Physician Needs to Know
1. Referral should be made to a hepatologist or hepatobiliary surgeon as adenomas are often treated due to the risk of hemorrhage and small risk of malignant transformation.
2. Checking a serum AFP level may be helpful to distinguish between hepatocellular cancer and adenoma. AFP is often elevated in patients with hepatocellular cancer but is not elevated in patients with adenomas.
Answers
1. Hepatocellular cancer may contain fat that is detectable at out-of-phase imaging. Hepatic lipomas and angiomyolipomas are relatively rare hepatic tumors that contain gross fat visible as low-attenuation material at CT or detectable based on signal loss seen in MR sequences obtained with fat saturation.
2. No. Bile ductules are not present in adenomas. By comparison, bile ductules are present in areas of FNH. The absence of bile ductules is a diagnostic criteria used at histology.
REFERENCES
1. Elsayes KM, Narra VR, Yin Y, Mukundan G, Lammle M, Brown JJ. Focal hepatic lesions: diagnostic value of enhancement pattern approach with contrast-enhanced 3D gradient-echo MR imaging. Radiographics 2005;25:1299-1320.
2. Grazioli L, Federle MP, Brancatelli G, Ichikawa T, Olivetti L, Blachar A. Hepatic adenomas: imaging and pathologic findings. Radiographics 2001;21:877-892.
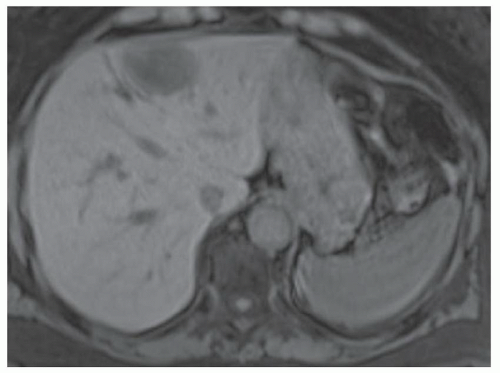
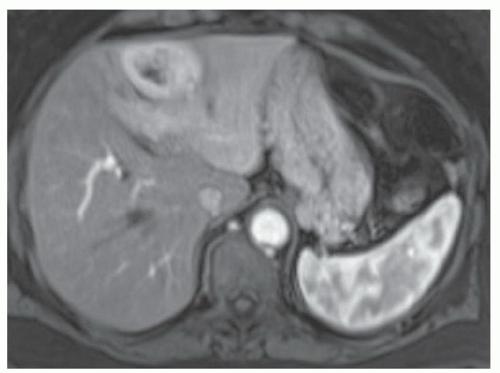
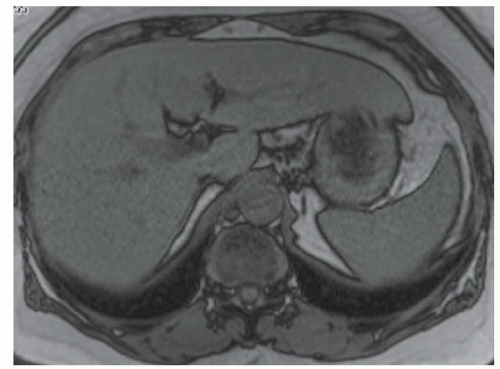
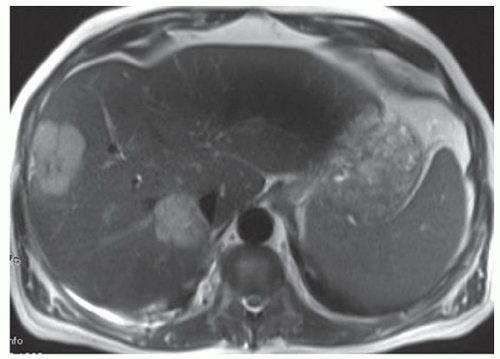
CASE 3.9 CLINICAL HISTORY 41-year-old woman with liver lesion seen at ultrasound performed because of elevated liver function tests. MR performed for further evaluation.
FINDINGS Axial T2-weighted (A), T1-weighted precontrast (B), T1-weighted arterial phase (C), T1-weighted portal venous phase (D), and T1-weighted delayed phase (E) MR images through the upper abdomen demonstrate a 6-cm, lobulated left hepatic lobe lesion. A T2 bright central scar is visible (A). The lesion hyperenhances in the late arterial phase (C, 20-second delay), but is “stealth” or isointense to the surrounding liver parenchyma at T2-weighted (A), T1-weighted precontrast (B), venous phase (D, 70-second delay), and delayed (E, 3-minute delay) images. Delayed enhancement of the central scar is visible (E) (contrast agent: Gd-BOPTA [MultiHance; Bracco Diagnostics, Milan, Italy]). Color Doppler ultrasound image (F) demonstrates a hypoechoic mass with a central blood vessel.
DIFFERENTIAL DIAGNOSIS Focal nodular hyperplasia (FNH), hepatocellular adenoma, hepatocellular carcinoma, hemangioma, hypervascular metastasis.
DIAGNOSIS FNH.
DISCUSSION FNH is a benign hepatic tumor and is the second most common liver tumor following hemangioma. FNH is usually asymptomatic and has no malignant potential. Though the etiology of FNH is uncertain, a vascular malformation or vascular injury has been posited as the underlying event leading to the development of FNH.
At gross pathology, malformed vascular structures are visible within the central scar. Lobules of hepatic parenchyma surround the central scar and are separated by radiating fibrous bands. FNH does not have a capsule.
At MR imaging, FNH is most commonly iso- or mildly hyperintense on T2-weighted images and iso- or mildly hypointense on T1-weighted precontrast images. The central scar is frequently bright on T2-weighted images due to the presence of blood vessels, myxomatous elements, and biliary ductules. FNH avidly and homogeneously enhances in the arterial phase and becomes more isointense in the portal venous phase. The fibrous component of the central scar frequently demonstrates delayed contrast uptake. At 1- to 3-hour delayed images performed after administration of Gd-BOPTA, FNH appears iso- to hyperintense to the surrounding liver in more than 96% of cases.
At CT imaging, FNH is most commonly iso- to hypoattenuating relative to the surrounding liver at precontrast imaging, avidly and homogeneously enhances in the arterial phase, and becomes more “stealth” or isoattenuating to the surrounding liver in the portal venous phase. As with MR, the fibrous component of the central scar may demonstrate delayed contrast uptake.
Ultrasound imaging features of FNH are nonspecific. At ultrasound, FNH is usually hypoechoic and may demonstrate a central blood vessel.
Questions for Further Thought
1. How can you differentiate this tumor from hepatocellular adenoma, hemangioma, and hepatocellular carcinoma?
View Answer
1. Helpful discriminators include the above-described and illustrated enhancement pattern, absence of a capsule (both hepatocellular carcinoma and adenoma frequently demonstrate capsules), and normal background liver parenchyma (hepatocellular carcinoma most frequently occurs in cirrhotic livers). The presence of a central scar is a nonspecific finding as T2 bright central scars can be seen in hemangiomas (but hemangiomas classically demonstrate peripheral nodular enhancement with centripetal fill-in rather than homogeneous enhancement) and hepatocellular carcinomas (scar is often dark on T1 and T2).
2. What is the usual management of FNH?
View Answer
2. As these tumors have no malignant potential, they are not resected. If the diagnosis of FNH can be made with confidence at imaging, biopsy is not required.
Reporting Responsibilities
1. Describe the location and size of the lesion.
2. Attempt to confidently characterize the lesion as FNH. When the diagnosis of FNH can be made with confidence at imaging, it is a “don’t touch” lesion that does not warrant biopsy or surgical resection.
What the Treating Physician Needs to Know
1. Whether you are confident that the lesion is an FNH.
2. The size and location of the lesion.
Answers
1. Helpful discriminators include the above-described and illustrated enhancement pattern, absence of a capsule (both hepatocellular carcinoma and adenoma frequently demonstrate capsules), and normal background liver parenchyma (hepatocellular carcinoma most frequently occurs in cirrhotic livers). The presence of a central scar is a nonspecific finding as T2 bright central scars can be seen in hemangiomas (but hemangiomas classically demonstrate peripheral nodular enhancement with centripetal fill-in rather than homogeneous enhancement) and hepatocellular carcinomas (scar is often dark on T1 and T2).
2. As these tumors have no malignant potential, they are not resected. If the diagnosis of FNH can be made with confidence at imaging, biopsy is not required.
REFERENCES
1. Hussain SM, Terkivatan T, Zondervan PE, et al. Focal nodular hyperplasia: findings at state-of-the-art MR imaging, US, CT and pathologic analysis. Radiographics 2004;24:3-17.
2. Silva AC, Evans JM, McCullough AE, et al. MR imaging of hypervascular liver masses: a review of current techniques. Radiographics 2009;29:385-402.
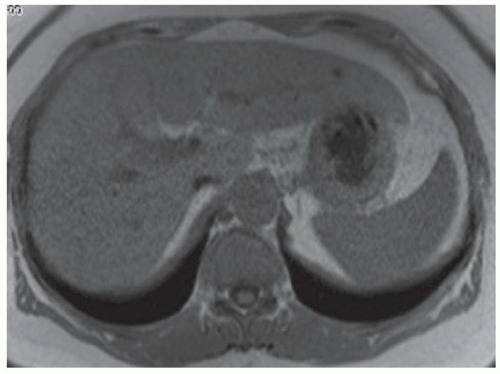
CASE 3.10 CLINICAL HISTORY 74-year-old woman presented to the emergency department with nausea and abdominal pain. CT (not shown) demonstrated a liver lesion. MR obtained for further characterization.
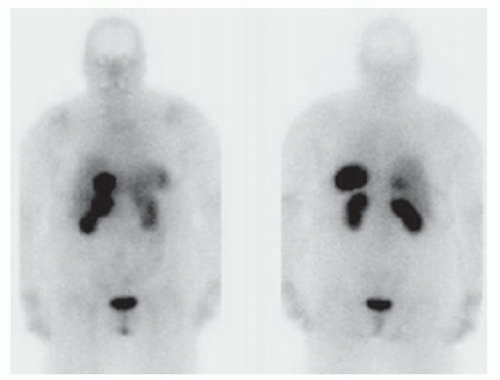
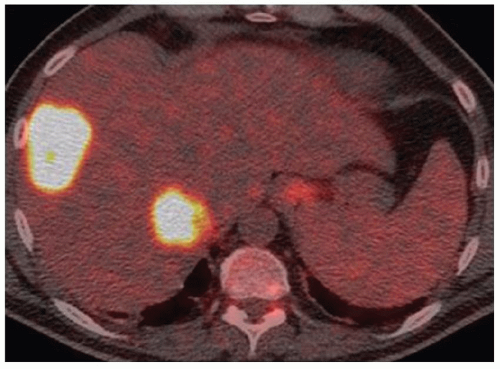
FINDINGS Axial T1-weighted precontrast (A) and postcontrast arterial phase (B) images demonstrate a T1 dark, hyperenhancing lesion in segment 4A. No signal loss was seen in out-of-phase images to suggest fat within the lesion (not shown). T2-weighted image (C) demonstrates intrinsic T2 bright signal in the lesion (contrast agent: Gd-BOPTA [MultiHance; Bracco Diagnostics, Milan, Italy]). Octreotide scan (D) demonstrates focal uptake of the radiopharmaceutical within the segment 4A lesion as well as in the region of the duodenum.
DIFFERENTIAL DIAGNOSIS BASED ON MAGNETIC RESONANCE IMAGES FNH, hemangioma, hepatocellular adenoma, hepatocellular carcinoma, hypervascular metastasis (e.g., primary neuroendocrine tumor, thyroid cancer, renal cell carcinoma, melanoma, and some breast cancers).
DIAGNOSIS Metastatic neuroendocrine tumor.
DISCUSSION The differential diagnosis of a hypervascular liver lesion includes metastatic disease from a hypervascular primary malignancy, hepatocellular carcinoma, hepatocellular adenoma, and FNH. Hemangiomas are also hyperenhancing lesions but classically demonstrate peripheral discontinuous nodular enhancement with central fill-in unlike the lesion in this case which is more homogeneously enhancing in the arterial phase.
Hepatocellular carcinoma would be unusual in a patient without a cirrhotic/nodular morphology of the liver. A history of oral contraceptive use and fat within the lesion (which this lesion did not have) would be more suggestive of hepatocellular adenoma. FNH would be expected to be more “stealth” or isointense on T1- and T2-weighted images.
This patient ultimately underwent a nuclear medicine octreotide scan that was positive for neuroendocrine tumor. A multilobulated duodenal bulb mass was seen at esophagoduodenoscopy and was determined to be the site of primary malignancy.
Question for Further Thought
1. Name different types of neuroendocrine tumors.
View Answer
1. Carcinoid tumors, gastrinomas, and somatostatinomas are different types of neuroendocrine tumors.
Reporting Responsibilities
1. Attempt to narrow differential diagnosis of hypervascular liver lesion.
2. If metastasis is a possibility, evaluate for the site of primary malignancy.
What the Treating Physician Needs to Know
1. If the differential diagnosis cannot be narrowed based on imaging features, suggest appropriate confirmatory testing such as the octreotide scan in this case.
2. If the site of primary malignancy can be identified.
Answer
1. Carcinoid tumors, gastrinomas, and somatostatinomas are different types of neuroendocrine tumors.
REFERENCE
1. Chang S, Choi D, Lee SJ, et al. Neuroendocrine neoplasms of the gastrointestinal tract: classification, pathologic basis and imaging features. Radiographics 2007;27:1667-1670.
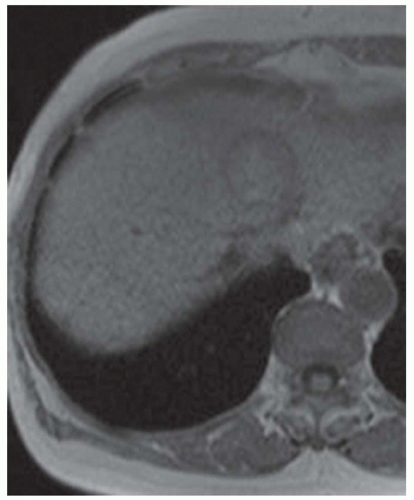
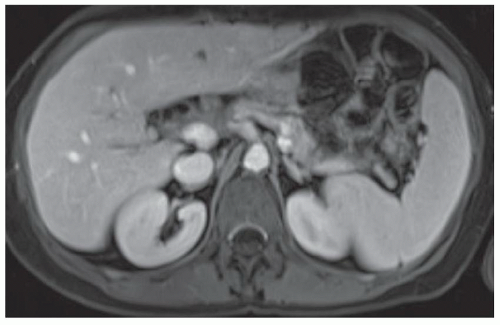
CASE 3.11 CLINICAL HISTORY 80-year-old man with hepatitis B and serum α-fetoprotein (AFP) level of 710 ng/mL.
FINDINGS In- (A) and out-of-phase (B) images demonstrate a 3.5-cm mass (arrows) in the segment 4a liver that loses signal in the out-of-phase images indicating that the mass contains intracellular lipid. The mass is T2 bright (C), hyperenhances in the arterial phase (D), and washes out with a capsule (E) in the delayed phase images. At color Doppler ultrasound, the mass is hyperechoic and heterogeneous. No discrete blood vessels could be identified in the mass at color Doppler ultrasound. This mass was new when compared with an MR from 6 months prior (not shown). Incidentally noted in the T2-weighted image is a 1.2-cm T2 bright structure (C) that had imaging features compatible with a hemangioma at postcontrast imaging (not shown) (contrast agent: Gd-BOPTA [MultiHance; Bracco Diagnostics, Milan, Italy]). Color Doppler ultrasound (F) demonstrates a corresponding echogenic mass.
DIFFERENTIAL DIAGNOSIS FNH, hemangioma, hepatocellular adenoma, hepatocellular carcinoma, metastasis from a hypervascular primary malignancy.
DIAGNOSIS Hepatocellular carcinoma.
DISCUSSION The differential diagnosis of a hyperenhancing hepatic mass is given above. Hemangioma can be eliminated from the list as the above-described mass does not demonstrate the classic peripheral discontinuous nodular enhancement with fill-in nor the T2 fluid bright signal seen with hemangiomas. FNH can be eliminated as the mass is not “stealth” in the T2-weighted image. Both hepatocellular adenoma and hepatocellular carcinoma may contain visible intracellular lipid though the patient demographics are usually quite different. Hepatic adenomas are rare tumors occurring usually in young women taking oral contraceptive pills, individuals with a glycogen storage disease, or individuals using anabolic steroids. Hepatocellular carcinomas tend to occur in patients with chronic liver disease like this patient.
Hepatocellular cancers are often bright at T2-weighted imaging. Fat-containing hepatocellular cancers will lose signal at out-of-phase imaging. About 80% to 90% of hepatocellular carcinomas are hypervascular and hyperenhance relative to the adjacent hepatic parenchyma in the arterial phase of imaging. About 10% to 20% of hepatocellular carcinomas are relatively hypoenhancing at arterial phase imaging. Hepatocellular carcinomas demonstrate a capsule at histologic evaluation in 65% to 82% of cases, and washout with a capsule is often seen at imaging.
Questions for Further Thought
1. What conditions can result in an elevated AFP level?
View Answer
1. A variety of tumors can produce elevated serum AFP levels including cancers of the pancreas, stomach, and biliary tree. Liver inflammation due to chronic liver disease can also result in an elevated AFP that increases with increasing inflammation.
2. Can the AFP level be normal in patients with hepatocellular cancer?
View Answer
2. Up to 30% of patients with hepatocellular cancer may have a normal AFP at the time of diagnosis. An AFP level >400 to 500 ng/mL is thought to be diagnostic of hepatocellular cancer.
Reporting Responsibilities
1. Describe the size, number, and location of lesions that are compatible with hepatocellular cancer.
2. Notify the ordering clinician if the finding is unexpected.
What the Treating Physician Needs to Know
1. In patients with imaging features compatible with hepatocellular cancer (as in this case), biopsy is NOT recommended to confirm the diagnosis as biopsy may result in track seeding.
2. If the patient is suitable for liver transplant, the patient should be referred to a transplant center as liver transplant is the preferred treatment for patients with cirrhosis and hepatocellular cancer who satisfy transplant criteria (a single tumor ≤5 cm in size or not more than three tumors ≤3 cm in size).
Answers
1. A variety of tumors can produce elevated serum AFP levels including cancers of the pancreas, stomach, and biliary tree. Liver inflammation due to chronic liver disease can also result in an elevated AFP that increases with increasing inflammation.
2. Up to 30% of patients with hepatocellular cancer may have a normal AFP at the time of diagnosis. An AFP level >400 to 500 ng/mL is thought to be diagnostic of hepatocellular cancer.
REFERENCES
1. Hanna RF, Aguirre DA, Kased N, Emery SC, Peterson MR, Sirlin CB. Cirrhosis-associated hepatocellular nodules: correlation of histopathologic and MR imaging features. Radiographics 2008;28:747-769.
2. Bialecki ED, Bisceglie AM. Diagnosis of hepatocellular cancer. HPB (Oxford) 2005;7:26-34.
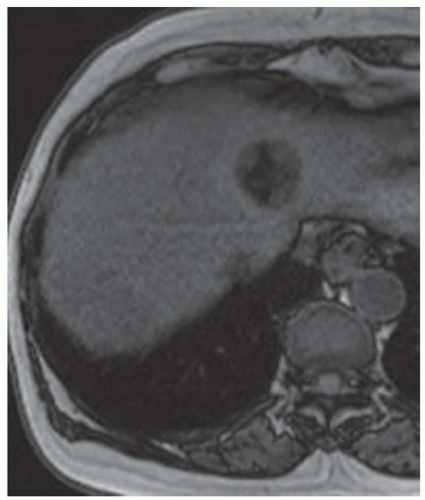
CASE 3.12 CLINICAL HISTORY 61-year-old woman with no known liver disease underwent noncontrast CT of the abdomen and pelvis to evaluate for kidney stones. No kidney stones were seen, but a liver mass was identified and MR was recommended for further evaluation.
FINDINGS Noncontrast T1-weighted MR (A) demonstrates a well-circumscribed 4.5-cm lesion in the segment 4 liver. T2-weighted MR (B) demonstrates corresponding T2 bright signal. This mass hyperenhances in the arterial phase (C) and washes out in the 3-minute delayed image (D). Comparing the in-phase (E) and out-of-phase image (F), small subtle areas of signal loss are seen in the out-of-phase image indicating a small fat component. Additionally, there is mild global signal loss in the out-of-phase image indicating mild diffuse hepatic steatosis (contrast agent: Gd-BOPTA [Multi- Hance; Bracco Diagnostics, Milan, Italy]).
DIFFERENTIAL DIAGNOSIS Hepatocellular adenoma, hepatocellular cancer, dysplastic nodule.
DIAGNOSIS Hepatocellular cancer.
DISCUSSION This tumor demonstrates the classic findings of hepatocellular cancer: arterial phase hyperenhancement, washout with a capsule at delayed phase imaging, T2 bright signal abnormality, and internal fat. Hepatic adenoma could have a very similar appearance. However, hepatocellular carcinoma is more likely in the above case given that the background liver exhibits early changes of chronic liver disease including a slightly nodular morphology and also linear areas of delayed reticular hyperenhancement (not well seen in the above images) indicating mild fibrosis.
A dysplastic nodule may show T2 bright signal abnormality and arterial hyperenhancement but should not demonstrate washout with a capsule at delayed imaging. Once washout is present, the lesion has progressed to hepatocellular cancer.
The above patient tested negative for hepatitis B and hepatitis C. This patient underwent a left hepatectomy as it was thought that she would have sufficient remaining liver parenchyma following surgery. Additionally, given that this patient’s liver synthetic function was normal, she likely would not have qualified for a liver transplant based on Model for End-Stage Liver Disease (MELD) score, even given exception points for the presence of tumor.
Questions for Further Thought
1. What is the purpose of the MELD score?
View Answer
1. The MELD score provides an estimate of 90-day mortality. In the United States, donor livers are allocated to the matching recipient with the highest MELD score.
2. What are the components of the MELD score?
View Answer
2. The MELD score is computed based on serum creatinine, bilirubin, and international normalized ratio (INR) levels. MELD scores can range from 6 to 40 with higher MELD scores reflecting more severe disease.
3. What are “MELD exception points”?
View Answer
3. MELD exception points are awarded to patients with cirrhosis and unresectable small hepatocellular cancers with “small tumors” defined as one tumor ≤5 cm or not more than three tumors ≤3 cm. This definition of “small tumors” is known as the Milan criteria as the original publication describing these criteria was from Milan, Italy.1 Individuals granted “exception points” begin with a MELD score of 22.
Reporting Requirements
1. Report that the mass is compatible with hepatocellular cancer.
2. Report that the patient has changes of mild hepatic steatosis and early changes of chronic liver disease.
What the Treating Physician Needs to Know
1. With technically optimized MR in experienced centers, hepatocellular cancer can be diagnosed based on imaging alone.
2. In general, biopsy of suspected hepatocellular cancers should not be performed given the risk of biopsy tract seeding.
Answers
1. The MELD score provides an estimate of 90-day mortality. In the United States, donor livers are allocated to the matching recipient with the highest MELD score.
2. The MELD score is computed based on serum creatinine, bilirubin, and international normalized ratio (INR) levels. MELD scores can range from 6 to 40 with higher MELD scores reflecting more severe disease.
3. MELD exception points are awarded to patients with cirrhosis and unresectable small hepatocellular cancers with “small tumors” defined as one tumor ≤5 cm or not more than three tumors ≤3 cm. This definition of “small tumors” is known as the Milan criteria as the original publication describing these criteria was from Milan, Italy.1 Individuals granted “exception points” begin with a MELD score of 22.
REFERENCES
1. Mazzaferro V, Regalia E, Doci R, et al. Liver transplantation for treatment of small hepatocellular carcinomas in patients with cirrhosis. N Engl J Med 1996;334:693-700.
2. Wald C, Russo MW, Heimbach JK, Hussain HK, Pomfret EA, Bruix J. New OPTN/UNOS policy for liver transplant allocation: standardization of liver imaging, diagnosis, classification, and reporting of hepatocellular carcinoma. Radiology 2013;266:376-382.
CASE 3.13 CLINICAL HISTORY 53-year-old man with hepatitis C undergoing screening for hepatocellular cancer.
FINDINGS Arterial phase T1 postcontrast MR (A) demonstrates a 1.8-cm hyperenhancing lesion in the segment 8/4A liver. This lesion washes out with peripheral rim enhancement in the 3-minute delayed image (B). No definite T2 correlate is seen in T2-weighted images with and without fat saturation (C, D). No signal loss is seen in this lesion in the out-of-phase (F) as compared with the in-phase (E) image to indicate fat. The patient’s liver is small and nodular indicating changes of chronic liver disease. Small-volume ascites is present (contrast agent: Gd-BOPTA [MultiHance; Bracco Diagnostics, Milan, Italy]).
DIFFERENTIAL DIAGNOSIS Dysplastic nodule, hepatocellular cancer, perfusional abnormality.
DIAGNOSIS Hepatocellular cancer.
DISCUSSION According to the Organ Procurement and Transplantation Network (OPTN) consensus panel (convened 2008, adopted 2011), lesions ≥1 and <2 cm can be diagnosed at MR as hepatocellular cancers if they demonstrate late arterial phase hyperenhancement, washout in more delayed images, and peripheral rim enhancement (capsule or pseudocapsule). The above 1.8-cm lesion therefore satisfies OPTN criteria for hepatocellular cancer as it hyperenhances in the late arterial phase and washes out with a capsule in the 3-minute delayed image.
Additionally, other criteria that can be used to diagnose hepatocellular cancer at MR according to OPTN include growth by ≥50% on serial scans ≤6 months apart and hyperenhancement in the late arterial phase for lesions that are ≥1 or <2 cm. For lesions ≥2 and ≤5 cm, hyperenhancement in the late arterial phase and either washout during later phases, or peripheral rim enhancement, or growth by ≥50% on scans ≤6 months apart is diagnostic of hepatocellular cancer.
Corresponding T2 bright signal abnormality and signal loss at out-of-phase imaging (indicating intravoxel fat) also may be seen in hepatocellular cancers. However, these latter two findings are not requirements to diagnose hepatocellular cancer at MR according to the OPTN.
Dysplastic nodules may hyperenhance in the late arterial phase and may demonstrate a T2 correlate but do not demonstrate washout in delayed images. Perfusional abnormalities generally demonstrate a geographic or triangular morphology rather than a round shape. Perfusional abnormalities are usually visible in the arterial phase but do not have correlates in the other series.
Available transplant livers go to the most ill patients as defined by the MELD score. MELD scores range from 6 to 40 with higher scores indicating more severely ill individuals. The MELD score is calculated based on creatinine, total bilirubin, and INR. When a donor liver becomes available, it usually goes to the matching recipient in a given region with the highest MELD score.
In 1996, a group from Milan, Italy (see Question 1), reported that liver transplant was an effective treatment for cirrhotic patients with small, unresectable hepatocellular cancers. Patients with hepatocellular cancers that fall within Milan criteria are given MELD exception points and begin with a MELD score of 22 rather than 6.
Question for Further Thought
1. What are the “Milan criteria”?
View Answer
1. The “Milan criteria” were first described in 1996 by Mazzaferro et al. in the New England Journal of Medicine. This landmark article concluded that liver transplantation was an effective treatment for patients with cirrhosis and small, unresectable tumors. Mazzaferro et al. defined “small tumors” as a single tumor ≤5 cm in diameter or not more than three tumors each measuring ≤3 cm in diameter. These categories have since become known as the “Milan criteria.”
Reporting Requirements
1. The above patient has a 1.8-cm hepatocellular cancer in the segment 8/4A liver.
2. The patient has a small, nodular liver indicating morphologic changes of chronic liver disease.
What the Treating Physician Needs to Know
1. The patient has a hepatocellular cancer based on MR criteria.
2. Biopsy should not be performed given the risk of needle tract seeding.
3. The patient’s tumor falls within Milan criteria, and the patient may be a candidate for liver transplant.
Answer
1. The “Milan criteria” were first described in 1996 by Mazzaferro et al. in the New England Journal of Medicine. This landmark article concluded that liver transplantation was an effective treatment for patients with cirrhosis and small, unresectable tumors. Mazzaferro et al. defined “small tumors” as a single tumor ≤5 cm in diameter or not more than three tumors each measuring ≤3 cm in diameter. These categories have since become known as the “Milan criteria.”
REFERENCES
1. Wald C, Russo MW, Heimbach JK, Hussain HK, Pomfret EA, Bruix J. New OPTN/UNOS policy for liver transplant allocation: standardization of liver imaging, diagnosis, classification, and reporting of hepatocellular carcinoma. Radiology 2013; 266:376-382.
2. Mazzaferro V, Regalia E, Doci R, et al. Liver transplantation for the treatment of small hepatocellular carcinomas in patients with cirrhosis. N Engl J Med 1996;334:692-699.
CASE 3.14 CLINICAL HISTORY 55-year-old man with hepatitis B undergoing MR screening for hepatocellular cancer.
FINDINGS T1 precontrast MR (A) demonstrates a subcentimeter area of low signal in the segment 5 liver adjacent to the gallbladder fossa. A larger rounded area of T2 bright signal abnormality is seen in this location (B). This lesion hyper-enhances in the arterial phase images (C) but does not wash out at delayed phase imaging (D). Note also morphologic features of chronic liver disease with a somewhat small, nodular liver and widening of the gallbladder fossa (contrast agent: Gd-BOPTA [MultiHance; Bracco Diagnostics, Milan, Italy]).
DIFFERENTIAL DIAGNOSIS Dysplastic nodule, hepatocellular cancer, perfusional abnormality.
DIAGNOSIS Dysplastic nodule.
DISCUSSION In the United States, approximately 80% to 90% of hepatocellular cancers occur in patients with cirrhosis. Hepatitis C infection is the most common cause of cirrhosis in North America and Europe, whereas hepatitis B infection is the most common cause of cirrhosis in Africa and Asia. Alcohol is the third leading cause of cirrhosis worldwide.
The liver responds to injury by regenerating. When subjected to prolonged injury (e.g., chronic viral hepatitis), innumerable regenerative nodules may form. It has been proposed that the development of hepatocellular cancer is often the end result of a process that begins with regenerative nodules which may progress to dysplastic nodules, and then may progress to hepatocellular cancer.
Diagnostic criteria for dysplastic nodules include hyper-enhancement at arterial phase imaging, sometimes with a T2 signal abnormality, but with no definite washout at delayed phase imaging. Arterial hyperenhancement with washout at delayed imaging is diagnostic of hepatocellular cancer. Additional features including T2 bright signal abnormality and internal fat are also supportive of a diagnosis of hepatocellular cancer but are not visible in every case.
A dysplastic nodule can be distinguished from a perfusional abnormality based on identification of a correlative area of signal abnormality in the T1 precontrast or T2-weighted images. Perfusional abnormalities are usually only visible in the arterial phase images without a correlate in the other series. Also, perfusional abnormalities often have a geographic rather than a rounded morphology. Many, though not all, perfusional abnormalities also occur in a subcapsular location.
The clinical management of nodules diagnosed as dysplastic nodules varies greatly when compared with those diagnosed as hepatocellular cancers at imaging. Perhaps because of lack of standardization in the imaging features used to diagnose a dysplastic nodule, the appropriate management of dysplastic nodules is a source of controversy in the literature. For example, recent guidelines from the American Association of Liver Disease do not recommend more aggressive screening of patients with dysplastic nodules. And the United Network for Organ Sharing (UNOS) does not require the reporting of dysplastic nodules at pretransplant imaging. However, high-volume transplant and imaging centers have observed a number of dysplastic nodules transition into hepatocellular cancers and recommend short-interval follow-up (e.g., 3-month MR) for patients with dysplastic nodules.
By comparison, hepatocellular cancers diagnosed at imaging usually prompt aggressive management ranging from resection in patients with small tumors and no known chronic liver disease, to liver transplant in patients with small, unresectable tumors and cirrhosis, to locoregional therapies such as chemoembolization in patients with unresectable hepatocellular cancer that does not meet criteria for liver transplant.
Question for Further Thought
1. What are the minimum MR sequences required for patients undergoing screening for hepatocellular cancer?
View Answer
1. Minimum MR sequence requirements include pre- and postcontrast T1-weighted images, T2-weighted images with and without fat saturation, and in- and out-of-phase images. Postcontrast images should be obtained in the late arterial, portal venous, and delayed phases of contrast material enhancement. Please refer to Wald et al.2 for further details regarding specific sequence parameters.
Reporting Requirements
1. Attempt to distinguish dysplastic nodules from hepatocellular cancers at imaging as management varies greatly.
2. Report whether the individual has features of chronic liver disease such as a small nodular liver, relative hypertrophy of the lateral segment left hepatic lobe and caudate lobe, and fissural widening which are morphologic features of chronic liver disease.
What the Treating Physician Needs to Know
1. Controversy exists with regard to management of dysplastic nodules. The American Association of Liver Disease and UNOS do not currently require more aggressive monitoring of or reporting of dysplastic nodules, respectively. However, high-volume transplant and imaging centers that use strict criteria for diagnosing dysplastic nodules (e.g., a hyperenhancing nodule ± a T2 correlate that does not wash out at delayed imaging) have observed a number of dysplastic nodules transition to hepatocellular cancer and recommend 3-month follow-up MR for dysplastic nodules.
2. Biopsy should not be performed to distinguish a dysplastic nodule from hepatocellular cancer, primarily because of the risk of biopsy tract seeding.
Answer
1. Minimum MR sequence requirements include pre- and postcontrast T1-weighted images, T2-weighted images with and without fat saturation, and in- and out-of-phase images. Postcontrast images should be obtained in the late arterial, portal venous, and delayed phases of contrast material enhancement. Please refer to Wald et al.2 for further details regarding specific sequence parameters.
REFERENCES
1. Hanna RF, Aguirre DA, Kased N, Emery SC, Peterson MR, Sirlin CB. Cirrhosis-associated hepatocellular nodules: correlation of histopathologic and MR imaging features. Radiographics 2008;28:747-769.
2. Wald C, Russo MW, Heimbach JK, Hussain HK, Pomfret EA, Bruix J. New OPTN/UNOS policy for liver transplant allocation: standardization of liver imaging, diagnosis, classification and reporting of hepatocellular cancer. Radiology 2013;266: 376-382.
CASE 3.15 CLINICAL HISTORY 58-year-old woman with hepatitis C virus undergoing screening for hepatocellular cancer.
FINDINGS Arterial phase image (A) demonstrates no suspicious liver lesions. However, at portal venous phase (not shown) and 3-minute delayed imaging (B) a 7-mm area of low signal is visible in the lateral segment left hepatic lobe. No correlate is visible in the T1 precontrast (C) or T2-weighted image (D). Comparing out-of-phase (E) and in-phase (F) images, a corresponding area of signal loss is visible in the in-phase image.
DIFFERENTIAL DIAGNOSIS Hepatocellular carcinoma, metastasis, siderotic nodule.
DIAGNOSIS Siderotic nodule.
DISCUSSION Siderotic nodules are iron-containing nodules. The key to making the correct diagnosis in this case is identifying the corresponding area of signal loss in the in-phase MR image. If in-phase images are acquired with a longer TE than out-of-phase images, susceptibility artifacts will “bloom” or look darker/bigger in the in-phase images. Susceptibility artifacts can be caused by a variety of substances including iron, air, and surgical clips and other postoperative changes. Identification of a corresponding area of signal loss in the in-phase image confirms the presence of a siderotic nodule.
Classic hepatocellular cancers “wash out” or appear of lower signal than the surrounding hepatic parenchyma at delayed phase imaging and could look similar to this siderotic nodule in the delayed phase image. However, a corresponding area of arterial hyperenhancement is usually necessary to confidently diagnose hepatocellular cancer at initial presentation. Other findings that are supportive of a diagnosis of hepatocellular cancer but are not visible in every case include a corresponding T2 bright signal abnormality and signal loss at out-of-phase imaging indicating the presence of fat.
A metastasis could have an identical appearance in the portal venous phase and delayed phase images. Therefore, evaluation of the in- and out-of-phase images is key to avoid mistakenly diagnosing a siderotic nodule as a metastasis. Likewise, assessing for signal loss at out-of-phase imaging is key to avoid the pitfall of mistakenly diagnosing focal fat deposition as a metastasis. Areas of focal fat will lose signal at out-of-phase imaging.
Question for Further Thought
1. What are other uses of in-phase images?
View Answer
1. As noted above, substances such as air and surgical clips that produce susceptibility artifact will “bloom” or look darker/bigger in in-phase images acquired with a TE that is longer than the out-of-phase images. Therefore, pneumobilia and pneumoperitoneum will be more conspicuous on in-phase images. Reviewing in-phase images is also one of the best ways to evaluate for changes indicating prior surgery such as susceptibility artifact in the subcutaneous tissues in the right upper quadrant in patients who have had a liver transplant, susceptibility artifact from clips in the gallbladder fossa in patients who have had a cholecystectomy, and susceptibility artifact about the gastric pouch in patients who have undergone gastric bypass surgery.
Reporting Requirements
1. The presence of siderotic nodules indicates that the patient has morphologic changes of chronic liver disease.
2. No differential diagnosis should be given for an entirely iron-containing siderotic nodule at MR.
3. Evaluate for sequelae of portal hypertension such as varices, ascites, and splenomegaly.
What the Treating Physician Needs to Know
1. Siderotic nodules indicate that the patient has morphologic changes of chronic liver disease. Serial MR surveillance to screen for hepatocellular cancer should be considered.
2. Siderotic nodules themselves do not require any specific treatment.
Answer
1. As noted above, substances such as air and surgical clips that produce susceptibility artifact will “bloom” or look darker/bigger in in-phase images acquired with a TE that is longer than the out-of-phase images. Therefore, pneumobilia and pneumoperitoneum will be more conspicuous on in-phase images. Reviewing in-phase images is also one of the best ways to evaluate for changes indicating prior surgery such as susceptibility artifact in the subcutaneous tissues in the right upper quadrant in patients who have had a liver transplant, susceptibility artifact from clips in the gallbladder fossa in patients who have had a cholecystectomy, and susceptibility artifact about the gastric pouch in patients who have undergone gastric bypass surgery.
REFERENCE
1. Hanna RF, Aguirre DA, Kased N, Emery SC, Peterson MR, Sirlin CB. Cirrhosis-associated hepatocellular nodules: correlation of histopathologic and MR imaging features. Radiographics 2008;28:747-769.
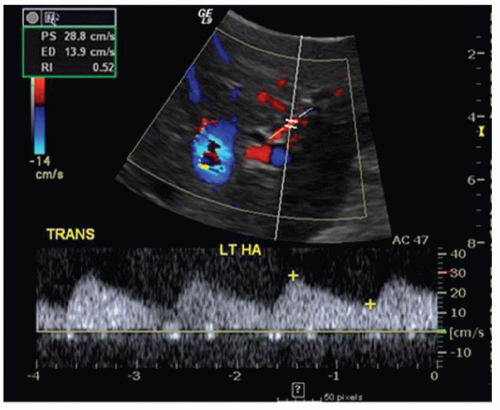
CASE 3.16 CLINICAL HISTORY 53-year-old woman 1 year status post liver transplant with abnormal liver function tests.
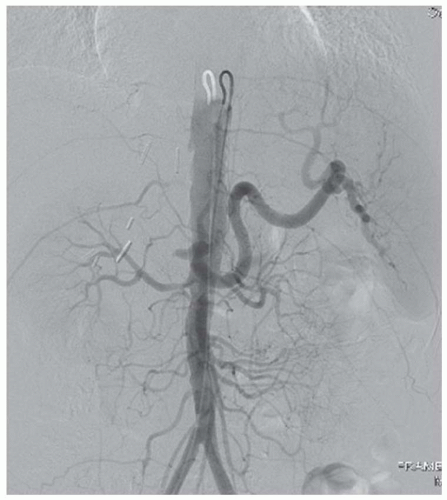
FINDINGS Ultrasound (A) was initially performed. A parvus tardus waveform with delayed upstroke and low-peak systolic velocity was noted in all hepatic arterial structures (A). Patient then underwent contrast-enhanced MR (B) that demonstrated occlusion of the hepatic artery approximately 1.5 cm beyond its origin. Confirmatory arteriogram (C) demonstrates hepatic artery occlusion approximately 1.5 cm beyond its origin. Note opacification of the splenic artery, renal arteries, superior mesenteric artery, and inferior mesenteric artery but no opacification of the hepatic artery.
DIFFERENTIAL DIAGNOSIS Hepatic artery occlusion.
DIAGNOSIS Hepatic artery occlusion.
DISCUSSION When evaluating a patient who has undergone liver transplant, evaluation of the patency of vascular structures is critical. As the hepatic artery provides the only supply to the intrahepatic biliary system, hepatic artery stenosis or thrombosis can result in biliary ischemia. If the arterial stenosis or thrombosis is not corrected, biliary ischemia can result in multiple nonanastomotic stenoses visible at imaging and ultimately be a cause of graft failure. When searching for the hepatic artery, remember that the donor hepatic artery may be anastomosed to the recipient hepatic artery or directly to the aorta via an interposition graft.
Liver transplants require anastomoses of the hepatic artery, portal vein, common bile duct, and inferior vena cava. In general, complications of liver transplant visible at imaging include vascular complications, biliary complications, and other complications. Vascular complications include stenosis or thrombosis at any of the three vascular anastomoses (hepatic artery, portal vein, or inferior vena cava). Pseudoaneurysms may also form at anastomotic sites or within the liver parenchymal as a complication of biopsy or other intervention. Biliary complications include anastomotic narrowing, bile leak, and cholangitis. Other complications include hematoma, abscess, recurrent hepatitis, recurrent malignancy, and post transplant lymphoproliferative disorder (PTLD).
The major indication for liver transplant is end-stage liver disease that will be cured by the procedure. Etiologies of end-stage liver disease that may warrant transplant are numerous and include viral hepatitis, alcoholic liver disease, non-alcoholic fatty liver disease (NAFLD), autoimmune hepatitis, liver malignancies (e.g., hepatocellular cancer and epithelioid hemangioendothelioma), metabolic liver diseases (e.g., Wilson disease, hereditary hemochromatosis, glycogen storage disease, and α1-antitrypsin deficiency), vascular diseases of the liver (e.g., Budd-Chiari syndrome), cholestatic liver diseases (e.g., primary biliary cirrhosis and PSC), and miscellaneous causes (e.g., hepatic trauma).
Contraindications for liver transplant include active substance abuse. No strict age cutoff exists though some centers will not transplant patients older than 60 years and other centers will not transplant patients older than 70 years.
Question for Further Thought
1. How do you explain the parvus tardus waveform seen at ultrasound whereas the hepatic artery appeared completely occluded at angiography?
View Answer
1. The ultrasound study was performed before MR and angiogram. It may be that the hepatic artery was stenotic at the time of ultrasound but became occluded by the time of the MR and angiogram. Alternatively, it may be that the intrahepatic arterial waveforms detected at ultrasound were perfused via small collateral vessels that had been recruited due to occlusion of the main hepatic artery.
Reporting Requirements
1. Contact the treating physician to inform him/her of the presence of hepatic artery occlusion.
2. Report that no biliary ductal dilatation is visible at the present time.
What the Treating Physician Needs to Know
1. The patient’s hepatic artery is occluded.
2. The transplant surgery service should be contacted to assess the patient for treatment/repair.
Answer
1. The ultrasound study was performed before MR and angiogram. It may be that the hepatic artery was stenotic at the time of ultrasound but became occluded by the time of the MR and angiogram. Alternatively, it may be that the intrahepatic arterial waveforms detected at ultrasound were perfused via small collateral vessels that had been recruited due to occlusion of the main hepatic artery.
REFERENCES
1. Singh AK, Nachiappan AC, Verma HA, et al. Postoperative imaging in liver transplantation: what radiologists should know. Radiographics 2010;30:339-351.
2. Varma V, Mehta N, Kumaran V, Nundy S. Indications and contraindications for liver transplantation. Int J Hepatol 2011; 121862 (Epublication).
CASE 3.17 CLINICAL HISTORY 62-year-old man with abnormal liver function tests.
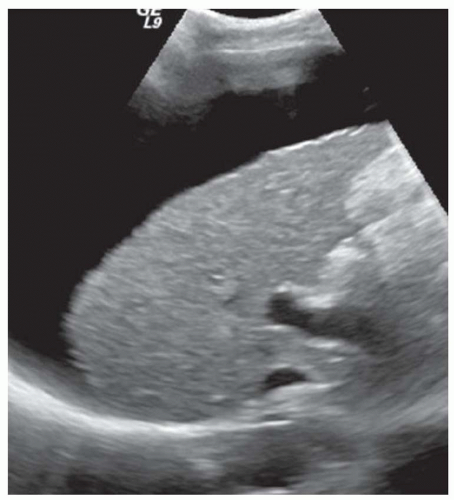
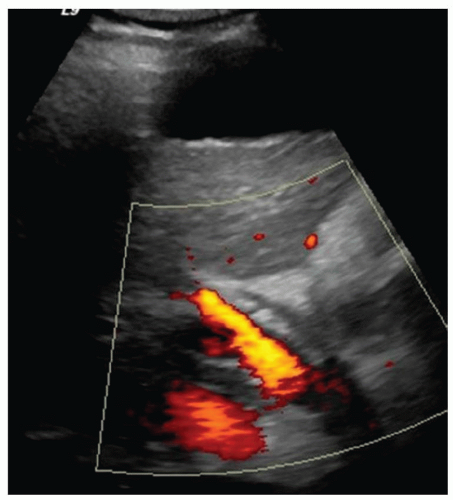
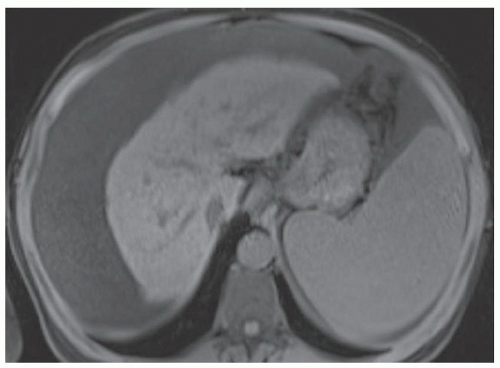
FINDINGS Grayscale ultrasound (A) demonstrates a small and nodular liver. Note the large amount of anechoic material surrounding the liver in keeping with ascites. Blood flow is detected in the main portal vein with power Doppler (B). T1- (C) and T2-weighted (D) MR also demonstrate a small and nodular liver with ascites as well as splenomegaly. (Evaluating signal intensity of the CSF is the most reliable way to determine if a sequence is T1- or T2-weighted. CSF is dark in T1-weighted images and is bright in T2-weighted images.)
DIFFERENTIAL DIAGNOSIS Hepatic cirrhosis with portal hypertension.
DIAGNOSIS Hepatic cirrhosis with portal hypertension.
DISCUSSION Cirrhosis is defined as liver disease characterized by hepatocyte damage and fibrosis. Cirrhosis is the seventh leading cause of disease-related death in the United States. Etiologies of cirrhosis include hepatitis C, hepatitis B, alcohol, and non-alcoholic fatty liver disease (NAFLD).
In early cirrhosis, liver morphology will appear normal at imaging. As cirrhosis progresses, a nodular hepatic contour is visible at imaging. Relative hypertrophy of the caudate lobe and of the lateral segment left hepatic lobe is also a morphologic feature of cirrhosis seen at imaging. Fissural widening, widening of the gallbladder fossa, and widening of the periportal space are also morphologic changes of cirrhosis.
As cirrhosis progresses, hepatic fibrosis results in increased pressure in the portal venous system, a condition known as portal hypertension. Findings seen in patients with portal hypertension include ascites, splenomegaly, and development of large collateral vessels. Large collateral vessels (also known as varices) form as a way for blood to return to the heart while bypassing the high-pressure hepatic portal venous system. Varices may form in a variety of locations, including around the esophagus, stomach, umbilicus, retroperitoneum, mesentery, rectum, and abdominal wall. Splenorenal shunts and hemorrhoids may also develop as collateral pathways in patients with portal hypertension.
As patients with cirrhosis are at increased risk for hepatocellular cancer, consideration should be given to hepatocellular cancer screening in patients with findings of chronic liver disease. Whether performed with CT or MR, arterial phase, venous phase, and delayed phase images are required for accurate hepatocellular cancer screening.
Questions for Further Thought
1. What percentage of patients who develop hepatocellular cancer have underlying cirrhosis?
2. What is the rate of development of hepatocellular cancer in patients with cirrhosis?
View Answer
2. Of patients with cirrhosis, approximately 1% to 4% will develop hepatocellular cancer per year.
Reporting Requirements
1. Report that the patient has morphologic findings of hepatic cirrhosis.
2. Report if the patient has findings of portal hypertension (e.g., splenomegaly, ascites, and varices).
What the Treating Physician Needs to Know
1. The above patient has findings of cirrhosis and portal hypertension.
2. Consideration should be given to screening for hepatocellular cancer.
Answers
1. Up to 90% of patients who develop hepatocellular cancer have underlying cirrhosis.
2. Of patients with cirrhosis, approximately 1% to 4% will develop hepatocellular cancer per year.
REFERENCES
1. Hussain SM, Reinhold C, Mitchell DG. Cirrhosis and lesion characterization at MR imaging. Radiographics 2009;29:1637-1652.
2. Brancatelli G, Federle MP, Ambrosini R, et al. Cirrhosis: CT and MR imaging evaluation. Eur J Radiol 2007;61:57-69.
CASE 3.18 CLINICAL HISTORY 53-year-old man with chronic liver disease undergoing screening for hepatocellular cancer.
FINDINGS T1-weighted MR (A) demonstrates a small, nodular liver in keeping with changes of advanced chronic liver disease. Notice also the geographic area of low signal with linear margins involving primarily segments 4A and 8 near the inferior vena cava. T2-weighted MR demonstrates corresponding T2 bright signal abnormality in segments 4A (B) and 8 (not shown). Dynamic contrast-enhanced MR demonstrates this area to be hypoenhancing relative to adjacent hepatic parenchyma in the arterial phase (C) of enhancement, hyperenhancing in the portal venous phase (D), and extremely hyperenhancing in the 3-minute delayed image (E) (contrast agent: Gd-BOPTA [MultiHance; Bracco Diagnostics, Milan, Italy]). Non-contrast CT (F) demonstrates a corresponding area of low attenuation.
DIFFERENTIAL DIAGNOSIS Confluent hepatic fibrosis.
DIAGNOSIS Confluent hepatic fibrosis.
DISCUSSION Hepatic fibrosis occurs in response to liver injury such as chronic inflammation due to hepatitis. Liver fibrosis is histologically defined as the deposition of substances including collagen and proteoglycans in the extracellular matrix.
The phrase “confluent fibrosis” refers to large, mass-like areas of fibrosis. Confluent fibrosis most commonly involves the anterior segment right hepatic lobe and medial segment left hepatic lobe.
At MR, areas of fibrosis usually demonstrate low T1 signal and high T2 signal due to the presence of water within areas of fibrosis. Areas of fibrosis exhibit delayed hyperenhancement at both CT and MR due to accumulation of gadolinium in the extracellular compartment. Areas of fibrosis can be thought of as hanging on to contrast material while contrast material washes out from the remainder of the liver. Delayed hyperenhancement in areas of confluent hepatic fibrosis is usually relatively homogeneous.
Confluent hepatic fibrosis can be distinguished from a neoplasm by its geographic morphology often with linear borders, associated capsular retraction, relatively homogeneous delayed hyperenhancement, and progressive volume loss over time.
Questions for Further Thought
1. What intrahepatic malignancy contains fibrous tissue and may demonstrate delayed hyperenhancement?
View Answer
1. Intrahepatic cholangiocarcinomas often demonstrate delayed hyperenhancement at contrast-enhanced MR due to the presence of fibrous tissue. Intrahepatic cholangiocarcinomas can be distinguished from areas of confluent fibrosis based on morphology. For example, intrahepatic cholangiocarcinomas usually have infiltrating or rounded borders, whereas confluent hepatic fibrosis is frequently geographic in shape with linear borders. Though intrahepatic cholangiocarcinomas often show delayed hyperenhancement, the enhancement is more heterogeneous than is seen with confluent hepatic fibrosis. Additionally, intrahepatic cholangiocarcinomas are often associated with biliary ductal dilatation more peripherally, whereas areas of confluent hepatic fibrosis are not.
2. List other fibrotic conditions elsewhere in the body that demonstrate delayed hyperenhancement.
View Answer
2. Retroperitoneal fibrosis and mesenteric fibrosis can also demonstrate delayed hyperenhancement due to the presence of fibrous tissue.
Reporting Requirements
1. Describe the presence of confluent hepatic fibrosis.
2. Describe any sequelae of portal hypertension such as splenomegaly, varices, and ascites.
3. Careful attention should be paid to evaluate for hepatocellular cancer in patients with findings of chronic liver disease.
What the Treating Physician Needs to Know
1. That the patient has areas of confluent hepatic fibrosis.
2. If the patient has findings of portal hypertension or evidence of hepatocellular cancer.
Answers
1. Intrahepatic cholangiocarcinomas often demonstrate delayed hyperenhancement at contrast-enhanced MR due to the presence of fibrous tissue. Intrahepatic cholangiocarcinomas can be distinguished from areas of confluent fibrosis based on morphology. For example, intrahepatic cholangiocarcinomas usually have infiltrating or rounded borders, whereas confluent hepatic fibrosis is frequently geographic in shape with linear borders. Though intrahepatic cholangiocarcinomas often show delayed hyperenhancement, the enhancement is more heterogeneous than is seen with confluent hepatic fibrosis. Additionally, intrahepatic cholangiocarcinomas are often associated with biliary ductal dilatation more peripherally, whereas areas of confluent hepatic fibrosis are not.
2. Retroperitoneal fibrosis and mesenteric fibrosis can also demonstrate delayed hyperenhancement due to the presence of fibrous tissue.
REFERENCES
1. Fein SC, Ganesan K, Mwangi I, et al. MR imaging of liver fibrosis: current state of the art. Radiographics 2009;29:1615-1635.
2. Elsayes KM, Narra VR, Yin Y, Mukundan G, Lammle M, Brown JJ. Focal hepatic lesions: diagnostic value of enhancement pattern approach with contrast-enhanced 3D gradient-echo MR imaging. Radiographics 2005;25:1299-1320.
CASE 3.19 CLINICAL HISTORY 56-year-old man with abdominal pain.
FINDINGS T1- (A) and T2-weighted (B) MR images demonstrate a T1 dark mass in the right hepatic lobe with intermediate- to-bright, heterogeneous T2 signal. Postcontrast MR images in the arterial (C) and portal venous (D) phase as well as 3-minute delayed (E) MR image demonstrate progressive enhancement of the mass (contrast agent: Gd-BOPTA [MultiHance; Bracco Diagnostics, Milan, Italy]).
DIFFERENTIAL DIAGNOSIS Hemangioma, hepatocellular carcinoma, intrahepatic cholangiocarcinoma.
DIAGNOSIS Intrahepatic cholangiocarcinoma.
DISCUSSION Cholangiocarcinoma is the second most common primary liver cancer following hepatocellular carcinoma. Cholangiocarcinomas are categorized based on location as extrahepatic, intrahepatic hilar, and intrahepatic peripheral.
At MR and CT imaging, intrahepatic cholangiocarcinomas demonstrate progressive enhancement at delayed imaging as seen in this case due to their fibrous nature. Progressive enhancement at delayed image is thought to reflect contrast material diffusing into the tumor interstitium.
By comparison, hepatocellular carcinomas classically demonstrate hyperenhancement in the arterial phase and washout at delayed imaging. An additional helpful discriminator is that cholangiocarcinomas tend to constrict adjacent vessels, whereas hepatocellular carcinomas more commonly invade adjacent vessels. Also, intrahepatic cholangiocarcinomas are often associated with more peripheral biliary ductal dilatation, whereas hepatocellular carcinomas are not. Hemangiomas demonstrate discontinuous peripheral nodular enhancement with gradual more central filling at delayed imaging.
Questions for Further Thought
1. What are risk factors for cholangiocarcinoma?
View Answer
1. Risk factors for cholangiocarcinoma include primary sclerosing cholangitis (PSC), choledochal cysts, and liver fluke infestation.
2. What tumor markers are elevated in patients with cholangiocarcinoma?
View Answer
2. Carcinoembryonic antigen (CEA) and carbohydrate antigen 19-9 (CA 19-9) are elevated in patients with cholangiocarcinomas but can also be elevated due to other etiologies. For example, CEA can be elevated in patients with colorectal cancer or cancers of the pancreas, lung, thyroid, or breast. CEA may also be elevated in the setting of infection and inflammatory bowel disease. CA 19-9 may also be elevated in the setting of pancreatic cancer, colorectal cancer, and gastric cancer. Nonmalignant obstructive jaundice can also result in an elevated CA 19-9.
Reporting Responsibility
1. Suggest cholangiocarcinoma in the differential diagnosis of an intrahepatic solid mass that demonstrates progressive enhancement at delayed imaging.
What the Treating Physician Needs to Know
1. Tumor size, location, and involvement of any major vascular structures.
Answers
1. Risk factors for cholangiocarcinoma include primary sclerosing cholangitis (PSC), choledochal cysts, and liver fluke infestation.
2. Carcinoembryonic antigen (CEA) and carbohydrate antigen 19-9 (CA 19-9) are elevated in patients with cholangiocarcinomas but can also be elevated due to other etiologies. For example, CEA can be elevated in patients with colorectal cancer or cancers of the pancreas, lung, thyroid, or breast. CEA may also be elevated in the setting of infection and inflammatory bowel disease. CA 19-9 may also be elevated in the setting of pancreatic cancer, colorectal cancer, and gastric cancer. Nonmalignant obstructive jaundice can also result in an elevated CA 19-9.
REFERENCES
1. Han JK, Choi BI, Kim AY, et al. Cholangiocarcinoma: pictorial essay of CT and cholangiographic findings. Radiographics 2002;22:173-187.
2. Ramage JK, Donaghy A, Farrant JM, et al. Serum tumor markers for the diagnosis of cholangiocarcinoma in primary sclerosis cholangitis. Gastroenterology 1995;108:865-869.
CASE 3.20 CLINICAL HISTORY 51-year-old man with pain.
FINDINGS Grayscale ultrasound image (A) demonstrates two heterogeneous, primarily hypoechoic masses in the right hepatic lobe. Color Doppler image (B) demonstrates blood flow within the posterior mass and also in the anterior mass (not shown). The masses are hypermetabolic at positron emission tomography CT imaging (C). T2-weighted MR (D) demonstrates two corresponding T2 bright areas of signal abnormality in the right hepatic lobe. The masses are dark in the T1 precontrast images (E) and hypoenhance at venous phase MR (F) (contrast agent: Gd-BOPTA [MultiHance; Bracco Diagnostics, Milan, Italy]).
DIFFERENTIAL DIAGNOSIS Abscesses, metastatic disease, lymphoma.
DIAGNOSIS Lymphoma (secondary, non-Hodgkin).
DISCUSSION Primary hepatic lymphoma is rare, accounting for less than 1% of extranodal primary lymphomas and approximately 1% of primary hepatic tumors. Primary hepatic lymphoma most commonly occurs in immunocompromised patients.
Secondary hepatic lymphoma is more common than primary hepatic lymphoma. Secondary lymphoma occurs in up to 50% of patients with non-Hodgkin lymphoma and up to 20% of patients with Hodgkin lymphoma. Secondary lymphoma is more commonly multifocal or diffusely infiltrating rather than a solitary lesion. Secondary lymphoma is usually found in patients with widespread lymphoma.
At ultrasound, hepatic lymphoma is usually hypoechoic to anechoic. At CT, hepatic lymphoma is usually hypoattenuating. At MR, hepatic lymphoma may be dark or bright on T2-weighted images, is usually dark on T1-weighted images, and is usually hypoenhancing.
Questions for Further Thought
1. What is the usual treatment for non-Hodgkin lymphoma?
2. How is the diagnosis of primary hepatic lymphoma usually made?
View Answer
2. As the imaging appearance of primary hepatic lymphoma is nonspecific, tissue sampling is usually required to make the diagnosis.
Reporting Responsibilities
1. Describe the size and location of the liver lesion(s).
2. Describe whether there are findings of extrahepatic disease.
What the Treating Physician Needs to Know
1. Whether the liver lesion(s) is an isolated finding or if there is other extranodal disease.
2. If the liver lesion is an isolated finding and demonstrates nonspecific imaging features, tissue sampling will likely be needed to make the diagnosis.
Answers
1. Chemotherapy is the primary treatment for non-Hodgkin lymphoma.
2. As the imaging appearance of primary hepatic lymphoma is nonspecific, tissue sampling is usually required to make the diagnosis.
REFERENCES
1. Elsayes KM, Narra VR, Yin Y, Mukundan G, Lammle M, Brown JJ. Focal hepatic lesions: diagnostic value of enhancement pattern approach with contrast-enhanced 3D gradient-echo MR imaging. Radiographics 2005;25:1299-1320.
2. Gazelle GS, Lee MJ, Hahn PF, et al. US, CT, and MRI of primary and secondary liver lymphoma. J Comput Assist Tomogr 1994;18:412-415.
3. Page RD, Romaguera JE, Osborne B, et al. Primary hepatic lymphoma: favorable outcome after combination chemotherapy. Cancer 2001;15:2023-2029.
4. Steller EJA, van Leeuwen MS, van Hillegersberg R, El Schipper M, Rinkes IHMB, Molenaar IQ. Primary lymphoma of the liver – a complex diagnosis. World J Radiol 2012;28:53-57.
CASE 3.21 CLINICAL HISTORY 68-year-old man with abnormal liver function tests 3 months after liver transplant.
FINDINGS Color Doppler ultrasound demonstrates heterogeneous hepatic parenchyma (A) and patent transplant vasculature (not shown). Patient next underwent MR imaging to better evaluate the liver parenchyma. T2-weighted MR (B) demonstrates innumerable 1- to 2-cm T2 bright liver lesions that are T1 dark (C) and hypoenhancing (D) (contrast agent: Gd-BOPTA [MultiHance; Bracco Diagnostics, Milan, Italy)].
DIFFERENTIAL DIAGNOSIS Abscesses, post-transplant lymphoproliferative disorder (PTLD), metastatic disease.
DIAGNOSIS PTLD.
DISCUSSION PTLD occurs as a complication of organ transplantation due to immunosuppression. A majority of cases appear to be related to Epstein-Barr virus. PTLD is a general term that describes a spectrum of illnesses. At the milder end of the spectrum, some patients will manifest an acute mononucleosis-type illness. At the more severe end of the spectrum, patients develop a monoclonal proliferation of lymphoid cells that meet diagnostic criteria for lymphoma. Liver biopsy was performed in the above patient, and pathology revealed B-cell lymphoma.
PTLD is uncommon occurring in approximately 1% to 2% of liver transplant patients. PTLD is slightly more common in other solid organ transplant patients (e.g., kidney and lung) and occurs in up to a third of patients who undergo bowel transplant or multiple organ transplants.
Of patients who develop PTLD, a majority of patients will develop PTLD in the first year after transplant when immunosuppression is most intense. At imaging, patients with PTLD may present with solid organ lesions or lymphadenopathy
with extranodal disease being more common. PTLD preferentially involves the transplanted organ. Central nervous system and gastrointestinal tract involvement also may occur. The mainstay of treatment of PTLD is cessation or decrease in immunosuppressive medications.
with extranodal disease being more common. PTLD preferentially involves the transplanted organ. Central nervous system and gastrointestinal tract involvement also may occur. The mainstay of treatment of PTLD is cessation or decrease in immunosuppressive medications.
Abscesses could have a similar appearance at imaging, though fever and elevated white blood cell count would be expected. Diffuse metastatic disease could have a similar appearance. Tissue sampling is usually required to make the diagnosis of PTLD.
Questions for Further Thought
1. What is the typical clinical presentation of PTLD?
View Answer
1. Clinical presentation of PTLD can be quite variable. Many patients are initially asymptomatic. However, given the propensity of PTLD to involve the transplanted organ or the gastrointestinal tract, evidence of graft dysfunction or diarrhea could reflect PTLD.
2. What are the most common locations of gastrointestinal involvement with PTLD?
View Answer
2. Distal small bowel and proximal colon are the most commonly affected bowel segments. PTLD appears similar to nontransplant-related lymphoma and may manifest as segmental bowel wall thickening with aneurysmal dilatation.
Reporting Requirements
1. If solid organ lesions or marked lymphadenopathy are seen in a transplant recipient, suggest the possibility of PTLD.
What the Treating Physician Needs to Know
1. Solid organ lesions or lymphadenopathy could reflect PTLD in a transplant recipient.
2. Tissue sampling will likely be needed to confirm the diagnosis.
Answers
1. Clinical presentation of PTLD can be quite variable. Many patients are initially asymptomatic. However, given the propensity of PTLD to involve the transplanted organ or the gastrointestinal tract, evidence of graft dysfunction or diarrhea could reflect PTLD.
2. Distal small bowel and proximal colon are the most commonly affected bowel segments. PTLD appears similar to nontransplant-related lymphoma and may manifest as segmental bowel wall thickening with aneurysmal dilatation.
REFERENCES
1. Borhani AA, Hosseinzadeh K, Almusa O, Furlan A, Nalesnik M. Imaging of posttransplantation lymphoproliferative disorder after solid organ transplantation. Radiographics 2009;29: 981-1000.
2. Shanbhogue AKP, Virmani V, Vikram R, et al. Spectrum of medication-induced complications in the abdomen: role of cross-sectional imaging. Am J Roentgenol 2011;197: W286-W294.
CASE 3.22 CLINICAL HISTORY 22-year-old man who underwent MR imaging of the chest, abdomen, and pelvis as part of evaluation as a potential renal transplant recipient. (At the authors’ institution, patients who are potential renal transplant recipients undergo screening MR image of the chest, abdomen, and pelvis to evaluate for soft-tissue neoplasms, patency of the deep pelvic veins, and for areas of stenosis involving the common iliac, external iliac, or common femoral arteries.)
FINDINGS Axial T1-weighted in-phase and out-of-phase images (A, B) through the upper abdomen demonstrate signal loss in the liver and spleen in the in-phase image. (The out-of-phase image can be identified based on the presence of the chemical shift artifact of the second kind, also known as the “India ink” artifact. This artifact occurs because the resonance frequencies of fat and water differ such that at TEs of approximately 2.3 ms at 1.5 T, fat and water protons are out-of-phase [imagine vectors pointing in opposite directions] and cancel each other out. A black line is seen at fat-water interfaces as a result of this cancellation in voxels that contain equal parts of fat and water.)
DIFFERENTIAL DIAGNOSIS Diffuse fat deposition, diffuse iron deposition.
DIAGNOSIS Diffuse iron deposition.
DISCUSSION Diffuse iron deposition is indicated by the signal loss in the liver and spleen seen in the in-phase image. This signal loss occurs because susceptibility artifacts due to iron increase with longer TEs, and the TE of the in-phase image (4.99 ms) is longer than the TE of the out-of phase image (2.33 ms).
Questions for Further Thought
1. How can you distinguish primary from secondary hemochromatosis?
View Answer
1. Patients with primary hemochromatosis (e.g., genetic hemochromatosis) develop iron deposition in the liver, pancreas, and myocardium with relative sparing of the spleen and bone marrow. Patients who develop secondary hemochromatosis (e.g., transfusional iron overload) develop iron deposition in the liver, spleen, and bone marrow, with relative sparing of the pancreas and myocardium.
2. What underlying diagnosis does this patient have?
View Answer
2. This is a challenging question, and if you answered it correctly you are to be congratulated. Note the atrophy/absence of the right rectus abdominis musculature. This patient has prune-belly syndrome also known as Eagle-Barrett syndrome (laxity/atrophy of the anterior abdominal wall, genitourinary abnormalities including hydronephrosis, and cryptorchidism) and developed renal failure due to long-standing hydronephrosis.
Reporting Responsibilities
1. Identify the presence of iron deposition.
2. Distinguish between primary and secondary hemochromatoses.
What the Treating Physician Needs to Know
1. That iron deposition is present.
2. If primary hemochromatosis is suspected based on the pattern of iron deposition, it should be specified in the report as early detection allows for treatment with phlebotomy prior to irreversible organ damage.
3. If there are findings of chronic liver disease such as a small, nodular liver.
Answers
1. Patients with primary hemochromatosis (e.g., genetic hemochromatosis) develop iron deposition in the liver, pancreas, and myocardium with relative sparing of the spleen and bone marrow. Patients who develop secondary hemochromatosis (e.g., transfusional iron overload) develop iron deposition in the liver, spleen, and bone marrow, with relative sparing of the pancreas and myocardium.
2. This is a challenging question, and if you answered it correctly you are to be congratulated. Note the atrophy/absence of the right rectus abdominis musculature. This patient has prune-belly syndrome also known as Eagle-Barrett syndrome (laxity/atrophy of the anterior abdominal wall, genitourinary abnormalities including hydronephrosis, and cryptorchidism) and developed renal failure due to long-standing hydronephrosis.
REFERENCES
1. Siegelman ES. Body MRI. Philadelphia, PA: Elsevier Saunders; 2005.
2. Woods AG, Brandon DH. Prune belly syndrome. A focused physical assessment. Adv Neonatal Care 2007;7:132-143.
CASE 3.23 CLINICAL HISTORY 67-year-old man with history of renal cell carcinoma, restaging examination.
FINDINGS Axial T1-weighted in-phase (A) and out-of-phase (B) images through the upper abdomen demonstrate signal loss in the liver in the out-of-phase image. (The out-of- phase image can be identified based on the presence of the chemical shift artifact of the second kind, also known as the “India ink” artifact. This artifact occurs because the resonance frequencies of fat and water differ such that at an echo time [TE] of approximately 2.2 ms at 1.5 T, fat and water protons are out-of-phase [imagine vectors pointing in opposite directions] and cancel each other out. A black line is seen at fat-water interfaces as a result of this signal cancellation in voxels that contain equal parts of fat and water.)
DIFFERENTIAL DIAGNOSIS Diffuse hepatic fat deposition, diffuse hepatic iron deposition.
DIAGNOSIS Diffuse hepatic fat deposition.
DISCUSSION Diffuse fat deposition is indicated by the signal loss in the liver seen in the out-of-phase image. Hepatic steatosis may result from a number of causes, including excessive alcohol consumption, obesity, insulin resistance, and steroid use. Nonalcoholic fatty liver disease (NAFLD) is a general term that encompasses both fatty liver (hepatic steatosis) and nonalcoholic steatohepatitis (NASH).
NASH is characterized by hepatic fat deposition with associated inflammation and can progress to fibrosis. NASH is an increasingly common etiology of chronic liver disease and cirrhosis in the United States. Individuals with hepatic steatosis may be asymptomatic or may have right upper quadrant pain or elevated liver function tests.
At cross-sectional imaging, hepatic inflammation manifests as heterogeneous arterial phase enhancement. Hepatic fibrosis is visible as reticular areas of increased enhancement at delayed postcontrast imaging.
Question for Further Thought
1. Name four characteristic areas of hepatic focal fat sparing and focal fat deposition.
View Answer
1. Along the (1) porta hepatis, (2) falciform ligament, (3) gallbladder fossa, and (4) subcapsular area.
Reporting Responsibilities
1. Describe the presence of hepatic fat deposition.
2. Describe whether there are findings of inflammation (heterogeneous arterial phase hyperenhancement) or fibrosis (delayed reticular hyperenhancement).
What the Treating Physician Needs to Know
1. Hepatic steatosis is present.
2. If there is evidence of acute inflammation.
3. If there is evidence of fibrosis.
Answer
1. Along the (1) porta hepatis, (2) falciform ligament, (3) gallbladder fossa, and (4) subcapsular area.
REFERENCE
1. Siegelman ES. Body MRI. Philadelphia, PA: Elsevier Saunders; 2005.
CASE 3.24 CLINICAL HISTORY 43-year-old woman with abnormal liver function tests.
FINDINGS Grayscale ultrasound (A) demonstrates an echogenic liver with a geographic hypoechoic area along the porta hepatis. No definite blood flow is visible in this area at color Doppler imaging (B). In-phase (C) and out-of-phase (D) MR demonstrate diffuse hepatic signal loss in the out-of-phase image with the exception of the hepatic parenchyma around the porta hepatis.
DIFFERENTIAL DIAGNOSIS Hepatic steatosis with fat sparing; hepatic steatosis with focal fat deposition.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree