Fig. 3.1
Proposed mechanism of liver regeneration by bone marrow cell (BMC) infusion
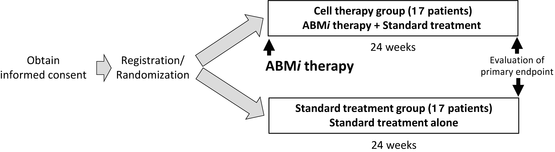
Fig. 3.2
Overview of “study on the efficacy and safety of autologous bone marrow cell infusion therapy (ABMi therapy) in HCV-related liver cirrhosis”
3.3.1 Inclusion Criteria
Patients with hepatitis C virus (HCV)-related liver cirrhosis
Patients with a Child-Pugh score of ≥7 (Child-Pugh B) at two times at least 90 days apart and in whom no improvement is expected with current medical treatment
Patients aged 20–75 years
Patients able to provide informed consent to participate in this study
3.3.2 Exclusion Criteria
Patients with liver cirrhosis due to another cause other than HCV or in whom the cause of liver cirrhosis is unknown
Patients with a current or past history of malignant neoplasm
Patients with gastroesophageal varices at risk of rupture
Patients with renal insufficiency and a serum creatinine ≥2 mg/dL
Patients with a hemoglobin <8 g/dL, a platelet count <50,000/μL, or a prothrombin time <40 %
Patients with a total bilirubin ≥3.0 mg/dL
Patients with a performance status of 3 or 4
Patients who refuse to consent to allogeneic blood transfusion
Patients in whom HBV infection, human immunodeficiency virus infection, viral infection with adult T-cell leukemia, or parvovirus B19 infection cannot be excluded
Women who are pregnant
Patients whom their attending physician deems are not suitable candidates for general anesthesia
Patients with a current or previous severe allergic reaction to a contrast agent
Any patient deemed unsuitable for study inclusion by their attending physician
3.3.3 Protocol
This study includes 34 patients who were randomly assigned to two groups: 17 patients each to the cell therapy group and standard treatment group. In the cell therapy group, autologous BM aspirates were obtained by a similar procedure as for BM transplants, which are widely used in the field of hematology.
Under general anesthesia, about 400 mL of autologous BM aspirate was obtained, and bone fragments were removed. The BM aspirates were concentrated and washed; then the BMCs were purified and concentrated according to standard operating procedures at the Center for Regenerative and Cell Therapy, which is a completely equipped Good Manufacturing Practice-grade facility. These BMCs were infused through a peripheral vein on the same day they were collected.
For 1 week after this procedure, each patient was in principle hospitalized for strict follow-up observation. If there were no problems, outpatient follow-up was then continued. Patients were followed up at least once monthly over a 6-month period, and treatment efficacy was assessed based on Child-Pugh scores.
3.3.4 Primary Endpoint
The primary endpoint was a rate of improvement of ≥1 in the Child-Pugh score at 24 weeks after cell therapy in the cell therapy group or 24 weeks after enrollment in the standard treatment group.
3.3.5 Secondary Endpoints
The following secondary endpoints were evaluated at 24 weeks after cell therapy in the cell therapy group or 24 weeks after enrollment in the standard treatment group:
1.
The rate of no worsening of the Child-Pugh score
2.
Changes in albumin levels
3.
Changes in serum fibrosis markers
4.
Changes in ascites volume
5.
Changes in improvement rates and disappearance rates of lower extremity edema
6.
Changes in subjective symptoms
7.
Incidence of adverse events
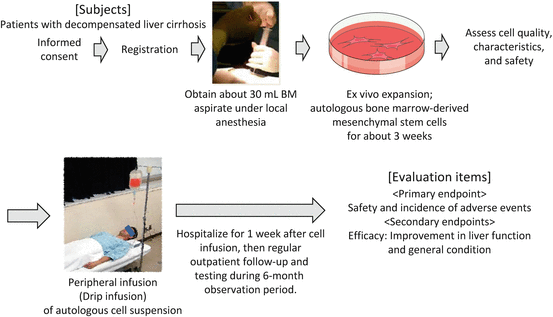
3.4 Our Less-Invasive Liver Regeneration Therapy Using Cultured Autologous Bone Marrow-Derived Mesenchymal Stem Cells (BMSCs)
Although studies on the effectiveness and safety of ABMi therapy are proceeding, many patients with decompensated liver cirrhosis are not candidates for general anesthesia, and thus many patients do not meet the indications for ABMi therapy. For patients with more advanced decompensated liver cirrhosis, less-invasive treatment must be developed.
Therefore, our hospital has conducted research on the use of autologous BMSCs cultured from small amounts of autologous BM aspirates (about 30 mL) obtained under local anesthesia. In our animal studies, the infusion of cultured human BMSCs improves hepatic fibrosis in severe combined immunodeficiency (SCID) mice with induced liver cirrhosis, indicating that clinical application is fully possible with this cell quantity [17]. Efficacy and safety have also been demonstrated in canine studies. As a result, a “study protocol to evaluate safety of less-invasive liver regeneration therapy using cultured autologous BMSCs in liver cirrhosis” was approved in August 2014. An overview of this clinical trial is discussed below and shown in Fig. 3.3.