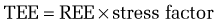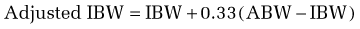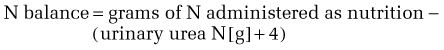CHAPTER 4 Nutritional Assessment and Management of the Malnourished Patient
BASIC NUTRITIONAL CONCEPTS
ENERGY STORES
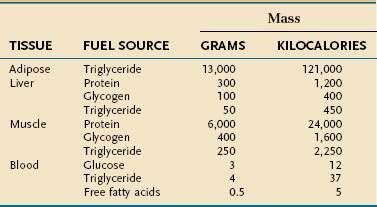
Endogenous energy stores are oxidized continuously for fuel. Triglyceride present in adipose tissue is the body’s major fuel reserve and is critical for survival during periods of starvation (Table 4-1). The high energy density and hydrophobic nature of triglycerides make it a fivefold better fuel per unit mass than glycogen. Triglycerides liberate 9.3 kcal/g when oxidized and are stored compactly as oil inside the fat cell. In comparison, glycogen produces only 4.1 kcal/g on oxidation and is stored intracellularly as a gel, containing approximately 2 g of water for every gram of glycogen. Adipose tissue is unable to provide fuel for certain tissues, such as bone marrow, erythrocytes, leukocytes, renal medulla, eye tissues, and peripheral nerves, which cannot oxidize lipids and require glucose for their energy supply. During endurance exercise, glycogen and triglycerides in muscle tissue provide an important source of fuel for working muscles.
ENERGY METABOLISM
Resting Energy Expenditure
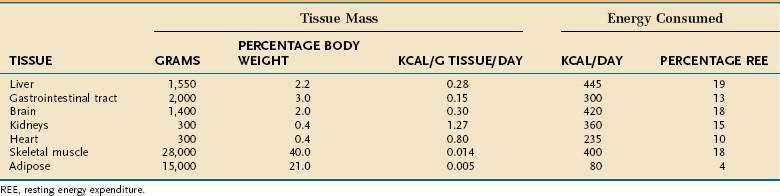
Resting energy expenditure (REE) represents energy expenditure while a person lies quietly awake in an interprandial state; under these conditions, approximately 1 kcal/kg body weight is consumed/hour in healthy adults. Energy requirements of specific tissues differ dramatically (Table 4-2). The liver, intestine, brain, kidneys, and heart constitute approximately 10% of total body weight but account for approximately 75% of REE. In contrast, skeletal muscle at rest consumes approximately 20% of REE but represents approximately 40% of body weight, and adipose tissue consumes less than 5% of REE but usually accounts for more than 20% of body weight.
Several empirical equations have been generated to estimate resting energy requirements (Table 4-3).1–4 These equations are useful in healthy subjects because they generate values that are usually within 10% of measured values. These equations are much less accurate, however, for persons who are at extremes in weight or who are ill, because alterations in body composition and metabolic stress influence energy expenditure. Protein-energy malnutrition and hypocaloric feeding without superimposed illness each decrease REE to values 10% to 15% below those expected for actual body size, whereas acute illness or trauma predictably increases energy expenditure (see later).
Table 4-3 Commonly Used Formulas for Calculating Resting Metabolic Rate
| Harris-Benedict Equation | |
|---|---|
| Men | 66 + (13.7 × W) + (5 × H) − (6.8 × A) |
| Women | 665 + (9.6 × W) + (1.8 × H) − (4.7 × A) |
| World Health Organization Formula | ||
|---|---|---|
| AGE (YR) | MALE | FEMALE |
| 0-3 | (60.9 × W) − 54 | (60.1 × W) − 51 |
| 3-10 | (22.7 × W) − 495 | (22.5 × W) + 499 |
| 10-18 | (17.5 × W) + 651 | (12.2 × W) + 746 |
| 18-30 | (15.3 × W) + 679 | (14.7 × W) + 996 |
| 30-60 | (11.2 × W) + 879 | (8.7 × W) + 829 |
| >60 | (13.5 × W) + 987 | (10.5 × W) + 596 |
A, age in years; H, height in centimeters; W, weight in kilograms.
Energy Expenditure of Physical Activity
The effect of physical activity on energy expenditure depends on the intensity and duration of daily activities. Highly trained athletes can increase their TEE 10- to 20-fold during athletic events. The activity factors shown in Table 4-4, each expressed as a multiple of REE, can be used to estimate TEE in active patients. The energy expended during physical activity is equal to REE × activity factor × duration of activity in hours/24 hours. TEE represents the summation of energy expended during all daily activities, including rest periods.
Table 4-4 Relative Thermic Effect of Various Levels of Physical Activity
| ACTIVITY LEVEL | EXAMPLES | ACTIVITY FACTOR |
|---|---|---|
| Resting | 1.0 | |
| Very light | Standing, driving, typing | 1.1-2.0 |
| Light | Walking 2-3 miles/hr shopping, light housekeeping | 2.1-4.0 |
| Moderate | Walking 3-4 miles/hr, biking, gardening, scrubbing floors | 4.1-6.0 |
| Heavy | Running, swimming, climbing, basketball | 6.1-10.0 |
Adapted from Alpers DA, Stenson WF, Bier DM. Manual of nutritional therapeutics. Boston: Little, Brown; 1995.
Recommended Energy Intake in Hospitalized Patients
Methods Incorporating Metabolic Stress Factors
Metabolic stress—that is, any injury or illness that incites some degree of systemic inflammation—will increase the metabolic rate through a variety of mechanisms (see later). The increase in energy expenditure is roughly proportional to the magnitude of the stress.5 Thus, the equations in Table 4-3 may be used to estimate the total energy requirement of an acutely ill patient by multiplying the predicted REE by a stress factor:
In acutely ill hospitalized patients, it is usually not necessary to include an activity factor. Although determination of the degree of stress is subjective, its use generates a caloric goal that closely approximates actual values. Table 4-5 delineates metabolic stress factors that accompany some common conditions and clinical scenarios in inpatients.
Table 4-5 Metabolic Stress Factors for Estimating Total Energy Expenditure in Hospitalized Patients
| INJURY OR ILLNESS | RELATIVE STRESS FACTOR* |
|---|---|
| Second- or third-degree burns, >40% BSA | 1.6-1.8 |
| Multiple trauma | 1.5-1.7 |
| Second- or third-degree burns, 20%-40% BSA | 1.4-1.5 |
| Severe infections | 1.3-1.4 |
| Acute pancreatitis | 1.2-1.4 |
| Second- or third-degree burns, 10%-20% BSA | 1.2-1.4 |
| Long bone fracture | 1.2 |
| Peritonitis | 1.2 |
| Uncomplicated postoperative state | 1.1 |
BSA, body surface area.
* A stress factor of 1.0 is assumed for healthy controls.
In using this formula, adjustments are necessary when the ABW is a misleading reflection of lean body mass. An adjusted ideal body weight should be substituted for ABW in obese individuals who are more than 30% heavier than their ideal body weight (desirable body weight, more commonly referred to as ideal body weight [IBW], appears in Table 4-6). The use of an adjusted IBW helps prevent an overestimation of energy requirements and is calculated as follows:
Method Without a Stress Factor
Table 4-7 outlines a simple method for estimating total daily energy requirements in hospitalized patients based on body mass index (BMI).6 With this method, energy expressed per kilogram is inversely related to BMI. Common sense needs to be applied when using any means to estimate energy expenditure in hospitalized individuals because illness commonly interjects artifacts into these calculations (e.g., ascites).
Table 4-7 Estimated Energy Requirements for Hospitalized Patients Based on Body Mass Index (BMI)*
| BODY MASS INDEX (BMI; KG/M2) | ENERGY REQUIREMENTS (KCAL/KG/DAY)† |
|---|---|
| <15 | 35-40 |
| 15-19 | 30-35 |
| 20-29 | 20-25 |
| ≥30 | 15-20 |
* The lower range within each BMI category should be considered in calculating energy requirements for insulin-resistant or critically ill patients to decrease the risk of hyperglycemia and infection associated with overfeeding.
† These values are recommended for critically ill patients and all obese patients; add 20% of the total calories when estimating energy requirements in non–critically ill patients.
Over the past two decades, the trend generally has been toward a more conservative approach to caloric delivery in acutely ill patients. One reason for this conservatism is that acute illness and its management often exacerbate preexisting diabetes or produces de novo glucose intolerance. As a result, hyperglycemia is a frequent consequence of enteral, and especially parenteral, nutrition. The issue seems to be particularly germane for intensive care unit (ICU) patients, in whom even modest hyperglycemia results in worse clinical outcomes, usually of an infectious nature. Clinical trials of high quality in surgical ICU (SICU)7 and medical ICU (MICU)8 patients have found that morbidity is substantially and significantly reduced in those randomized to intensive insulin therapy who maintained serum glucose levels below 111 mg/dL compared with those whose glucose values were maintained below 215 mg/dL. Mortality also was significantly lower in those in the SICU randomized to receive tight glucose control, although in the MICU study such reductions in mortality caused by tight glucose control only were realized in those residing in the MICU longer than three days. These clinical observations substantiate years of animal studies showing that even modest hyperglycemia impairs immune function in a variety of tissues.9 The clinical benefits of tight glucose control in the ICU, however, have not always been reproducible,10 and come at the cost of more frequent hypoglycemic episodes,7,8,10 so the issue of how tight glucose control should be remains controversial. Results of a recent meta-analysis of 29 trials in critically ill patients recapitulate the previously observed discrepancies between SICU and MICU patients.11 Overall, the relative risk of septicemia was reduced approximately 25% in those randomized to tight glucose control, but this salutary effect largely was attributable to the SICU patients, in whom the reduction in septicemia was almost 50%. In contrast, no benefit was observed in MICU patients. Also in this meta-analysis, no demonstrable benefit in overall mortality was evident in any of the categories of critically ill patients.
PROTEIN
Twenty different amino acids are found commonly in human proteins. Some amino acids (histidine, isoleucine, leucine, lysine, methionine, phenylalanine, threonine, tryptophan, valine, and possibly arginine) are considered essential because their carbon skeletons cannot be synthesized by the body. Other amino acids (glycine, alanine, serine, cysteine, cystine, tyrosine, glutamine, glutamic acid, asparagine, and aspartic acid) are nonessential in most circumstances because they can be made from endogenous precursors or essential amino acids. In disease states, nonessential amino acids may become essential—so-called conditionally essential amino acids. Thus, infectious morbidity and wound healing appear to be improved in critically ill patients by the inclusion of supplemental glutamine in total parenteral nutrition (TPN) because of cellular depletion of this amino acid. Studies to date suggest that the clinical benefits of providing supplemental quantities of parenteral glutamine only are realized in patients with particularly high severity of illness scores (e.g., high APACHE II or SOFA scores), although the true nature of its efficacy likely will remain controversial until the results of several ongoing clinical trials become known.12 Similarly, it has been shown that cysteine and tyrosine are essential in some patients with cirrhosis13 because of impaired hepatic synthesis.
The body of an average 75-kg man contains approximately 12 kg of protein. In contrast to fat and carbohydrate, there is no storage depot for protein, so excess intake is catabolized and the nitrogen component is excreted. Inadequate protein intake causes net nitrogen losses and, because no depot form of protein exists, there is an obligatory net loss of functioning protein. The U.S. Recommended Daily Allowance (RDA) of protein has been established at 0.8 g/kg/day, which reflects a mean calculated requirement of 0.6 g/kg/day plus an added factor to take into account the biological variance in requirement observed in a healthy population. Intravenously administered amino acids are as effective in maintaining nitrogen balance as oral protein of the same amino acid composition.14
Individual protein requirements are affected by several factors, such as the amount of nonprotein calories provided, overall energy requirements, protein quality, and the patient’s nutritional status (Table 4-8). Protein requirements increase when calorie intake does not meet energy needs. The magnitude of this increase is directly proportional to the deficit in energy supply. Therefore, nitrogen balance reflects protein intake and energy balance. Thus, correcting a negative nitrogen balance sometimes may be achieved merely by increasing the caloric delivery if the total amount of calories has been inadequate.
Table 4-8 Recommended Daily Protein Intake*
| CLINICAL CONDITION | DAILY PROTEIN REQUIREMENT (G/KG IBW) |
|---|---|
| Normal | 0.75 |
| Metabolic stress | 1.0-1.6 |
| Hemodialysis | 1.2-1.4 |
| Peritoneal dialysis | 1.3-1.5 |
IBW, ideal body weight.
* Additional protein requirements are needed to compensate for excess protein loss in specific patient populations (e.g., patients with burn injuries, open wounds, protein-losing enteropathy, or nephropathy). Lower protein intake may be necessary for patients with renal insufficiency not treated by dialysis and certain patients with liver disease and hepatic encephalopathy.
As metabolic stress (and with it, metabolic rate) increases, nitrogen excretion increases proportionately; quantitatively, the relationship is approximately 2 mg nitrogen (N)/kcal of REE. In part, this increase is explained by the fact that in metabolic stress, a larger proportion of the total substrate that is oxidized for energy is from protein. This has two important implications for managing the nutritional needs of ill patients. The first is that illness, by increasing catabolism and metabolic rate, increases the absolute requirement for protein (see Table 4-8), and does so in a manner that is roughly proportional to the degree of stress. Second, because a greater proportion of energy substrate in acute illness comes from protein, nitrogen balance is more readily achieved if a larger proportion of the total calories are from protein. In healthy adults, as little as 10% of total calories need to come from protein to maintain health, whereas in the ill patient, nitrogen balance is achieved more easily if 15% to 25% of total calories are delivered as protein.
Additional proteins are needed to compensate for excess loss in specific patient populations (e.g., patients with burn injuries, open wounds, and protein-losing enteropathy or nephropathy). Delivering less protein than is needed often is a necessary compromise in patients with acute renal insufficiency who are not adequately dialyzed, because in this situation the rise in azotemia is directly proportional to protein delivery. Once adequate dialysis is available, protein delivery should be increased to the actual projected need, including additional protein to compensate for losses resulting from dialysis (see Table 4-8). Most patients with hepatic encephalopathy respond to simple pharmacologic measures and therefore do not require a protein restriction; however, those who do not respond may benefit from a modest protein restriction (~0.6 g/kg/day).
CARBOHYDRATE
There is no absolute dietary requirement for carbohydrate because glucose can be synthesized from endogenous amino acids as well as glycerol. Nevertheless, carbohydrate is an important fuel because of the interactions between carbohydrate and protein metabolism. Carbohydrate intake stimulates insulin secretion, which inhibits muscle protein breakdown,15 stimulates muscle protein synthesis,16 and decreases endogenous glucose production from amino acids.17 In addition, glucose is the required or preferred fuel for red and white blood cells, the renal medulla, eye tissues, peripheral nerves, and the brain. Once glucose requirements for these tissues are met (∼150 g/day), however, the protein-sparing effects of carbohydrate and fat are similar.18
LIPIDS
Lipids consist of triglycerides (TGs), sterols, and phospholipids. These compounds serve as sources of energy, precursors for steroid hormone, prostaglandin, thromboxane, and leukotriene synthesis, structural components of cell membranes, and carriers of essential nutrients. Dietary lipids are composed mainly of TGs, which contain saturated and unsaturated long-chain fatty acids (FAs) of 16 to 18 carbons. The use of fat as a fuel requires the hydrolysis of endogenous or exogenous TGs and cellular uptake of released FAs. Long-chain FAs are delivered across the outer and inner mitochondrial membranes by a carnitine-dependent transport system.19 Once inside the mitochondria, FAs are degraded by beta oxidation to acetyl coenzyme A (CoA), which then enters the TCA cycle. Therefore, the ability to use fat as a fuel depends on normally functioning mitochondria. A decrease in the number of mitochondria or oxidative enzymes associated with aging20 or deconditioning favors the use of carbohydrate as fuel.21
Essential Fatty Acids
Humans lack the desaturase enzyme needed to produce the n-3 (double bond between carbons 3 and 4) and n-6 (double bond between carbons 6 and 7) FA series. Linoleic acid (C18 : 2, n-6) and linolenic acid (C18 : 3, n-3), therefore, should constitute at least 2% and 0.5%, respectively, of the daily caloric intake to prevent essential FA deficiency (EFAD). Before the advent of parenteral nutrition, EFAD was recognized only in infants and manifested as a scaly rash with a specific alteration in the plasma FA profile (see later). Adults previously were thought not to be susceptible to EFAD because of sufficient essential FA stores in adipose tissue. However, an abnormal FA profile in conjunction with a clinical syndrome of EFAD is now known to occur sometimes in adults with severe short bowel syndrome who are on long-term total parenteral nutrition (TPN) that lacks parenteral lipids.22 Adults who have moderate to severe fat malabsorption (fractional fat excretion >20%) from other causes and who are not TPN-dependent also frequently display a biochemical profile of EFAD,23 although whether such a biochemical state carries adverse clinical consequences with it is unclear. Moreover, TPN lacking any source of fat may lead to EFAD in adults if no exogenous source of EFAs is available; the plasma pattern of EFAD may be observed as early as 10 days after glucose-based TPN is started and before the onset of any clinical features.24 In this situation, EFAD is probably attributable to the increase in plasma insulin concentrations caused by TPN, because insulin inhibits lipolysis and therefore the release of endogenous essential FAs. The biochemical diagnosis of EFAD is defined as an absolute and relative deficiency in the two EFAs in the plasma FA profile. The full clinical EFAD syndrome includes alopecia, scaly dermatitis, capillary fragility, poor wound healing, increased susceptibility to infection, fatty liver, and growth retardation in infants and children.
MAJOR MINERALS
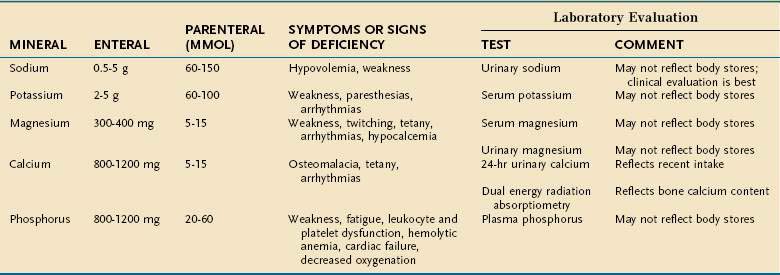
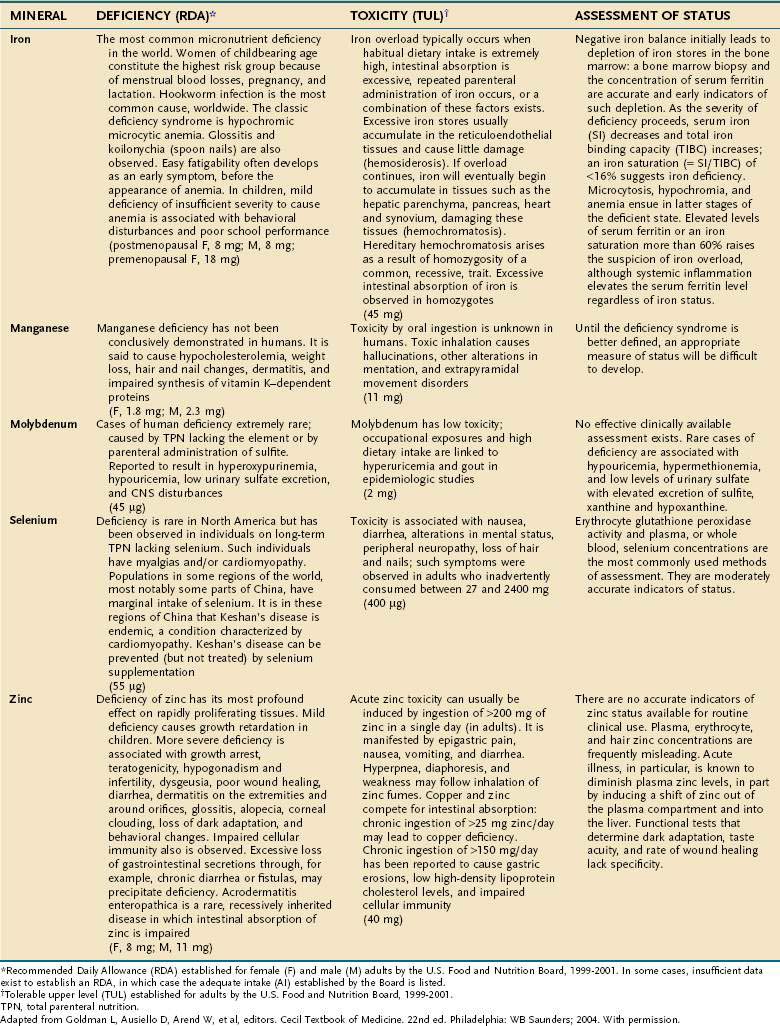
Major minerals are inorganic nutrients that are required in large (>100 mg/day) quantities, and are important for ionic equilibrium, water balance, and normal cell function. Malnutrition and nutritional repletion can have dramatic effects on major mineral balance. The evaluation of macromineral deficiency and recommended daily allowance (RDA) of minerals for healthy adults are shown in Table 4-9.
MICRONUTRIENTS
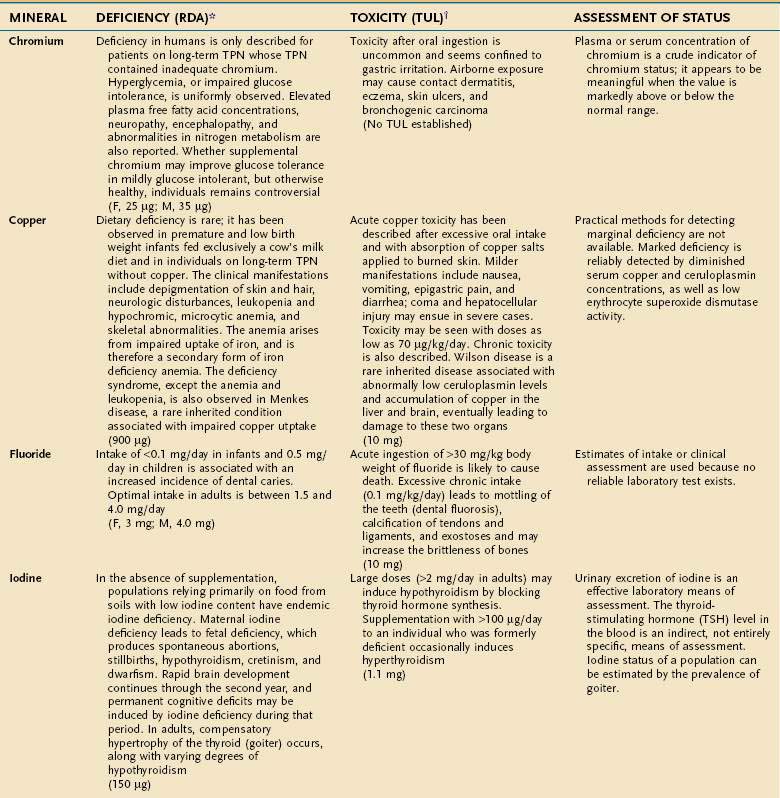
An individual’s dietary requirement for any given micronutrient is determined by many factors, including its bioavailability, the amount needed to sustain its normal physiologic functions, a person’s gender and age, any diseases or drugs that affect the nutrient’s metabolism, and certain lifestyle habits, such as smoking and alcohol use. The U.S. National Academy of Sciences Food and Nutrition Board regularly updates dietary guidelines that define the quantity of each micronutrient that is “adequate to meet the known nutrient needs of practically all healthy persons.” These RDAs underwent revision between 1998 and 2001, and the values for adults appear in Tables 4-10 and 4-11. The formulation of an RDA takes into account the biological variability in the population, and, therefore, RDAs are set 2 SDs above the mean requirement; this allows the requirements of 97% of the population to be met and ingestion of quantities that are somewhat less than the RDA usually are sufficient to meet the needs of a particular individual. A “tolerable upper limit (TUL),” which is “the maximal daily level of oral intake that is likely to pose no adverse health risks,” also has been established for most of the micronutrients (see Tables 4-10 and 4-11). Present recommendations for how much of each micronutrient is needed in individuals on TPN are based on far less data than what were available for the development of the RDAs. Nevertheless, it is important to have guidelines, and Table 4-12 provides such recommendations.
Table 4-12 Guidelines for Daily Delivery of Parenteral Micronutrients in Adults and Children
| MICRONUTRIENT | ADULTS | CHILDREN |
|---|---|---|
| Vitamin | ||
| A | 1000 µg (= 3300 IU) | 700 µg |
| D | 5 µg (= 200 IU) | 10 µg |
| E | 10 mg (= 10 IU) | 7 mg |
| K | 1 mg | 200 µg |
| C | 100 mg | 80 mg |
| Folate | 400 µg | 140 µg |
| Niacin | 40 mg | 17 mg |
| Riboflavin | 3.6 mg | 1.4 mg |
| Thiamine | 3 mg | 1.2 mg |
| B6 | 4 mg | 1.0 mg |
| B12 | 5 µg | 1.0 µg |
| Pantothenic acid | 15 mg | 5 mg |
| Biotin | 60 µg | 20 µg |
| Trace Elements | ||
| Copper | 0.5-1.5 mg | 20 µg/kg/day |
| Chromium | 10-15 µg | 0.2 µg/kg/day |
| Manganese | 0.1 mg | 1.0 µg/kg/day |
| Zinc | 2.5-4.0 mg | 50 µg/kg/day |
| Molybdenum | 15 µg | 0.25 µg/kg/day |
| Iodine* | — | — |
| Selenium | 100 µg | 2.0 µg/kg/day |
| Iron | 1-2 mg | 1 mg/day |
* Naturally occurring contamination of parenteral nutrition appears to provide sufficient quantities of iodine.
Adult vitamin guidelines adapted from American Society of Parenteral and Enteral Nutrition (ASPEN). Board of Directors and the Clinical Guidelines Task Force. Guidelines for the use of parenteral and enteral nutrition in adult and pediatric patients. JPEN J Parenter Enteral Nutr 2002;26:144. Children’s values adapted from Greene HL, Hambidge KM, Schanler R, Tsang RC. Guidelines for the use of vitamins, trace elements, calcium, magnesium, and phosphorus in infants and children receiving total parenteral nutrition: Report of the Subcommittee on Pediatric Parenteral Nutrient Requirements from the Committee on Clinical Practice Issues of the American Society for Clinical Nutrition. Am J Clin Nutr 1988; 48:1324; Am J Clin Nutr 1989; 49:1332; and Am J Clin Nutr 1989; 50:560.
VITAMINS
Vitamins are categorized as fat-soluble (A, D, E, K) or water-soluble (all others) (see Table 4-10). This categorization remains physiologically meaningful; none of the fat-soluble vitamins appear to serve as coenzymes, whereas almost all of the water-soluble vitamins appear to function in that role. Also, the absorption of fat-soluble vitamins is primarily through a micellar route, whereas the water-soluble vitamins are not absorbed in a lipophilic phase in the intestine (see Chapter 100).
TRACE MINERALS
Compelling evidence exists for the essential nature of 10 trace elements in humans—iron, zinc, copper, chromium, selenium, iodine, fluorine, manganese, molybdenum, and cobalt (see Table 4-11). The biochemical functions of trace elements have not been as well characterized as those of the vitamins, but most of their functions appear to be as components of prosthetic groups or as cofactors for a number of enzymes.
Aside from iron, the trace mineral depletion that clinicians are most likely to encounter is zinc deficiency. Zinc depletion is a particularly germane issue to the gastroenterologist because the gastrointestinal tract is a major site for zinc excretion. Chronically excessive losses of gastrointestinal secretions, such as chronic diarrhea in inflammatory bowel disease, is a known precipitant for zinc deficiency, and in this setting zinc requirements often increase several-fold.25 Nevertheless, a biochemical diagnosis of zinc deficiency is problematic, as is true for many of the other essential trace minerals. Accurate laboratory assessment of zinc status is complicated by the very low concentrations of zinc in bodily fluids and tissues, a lack of correlation between serum and red blood cell levels of zinc with levels in the target tissues, and the reality that functional tests have yet to be devised. Furthermore, it is well recognized that in acute illness a shift in zinc occurs from the serum compartment into the liver, further obscuring the diagnostic value of serum zinc levels.26,27 Thus, it is often best simply to proceed with empirical zinc supplementation in patients whose clinical scenario puts them at high risk of zinc deficiency.
Some reports have indicated that TPN solutions that deliver several-fold more manganese than what is recommended in Table 4-12 may lead to deposition of the mineral in the basal ganglia, with extrapyramidal symptoms, seizures, or both.28 Because the content of manganese varies widely in the different trace element mixtures available for TPN compounding, the health professional needs to be mindful of this issue as protocols for TPN admixtures are developed.
PHYSIOLOGIC AND PATHOPHYSIOLOGIC FACTORS AFFECTING MICRONUTRIENT REQUIREMENTS
Age
An evolution of physiology continues throughout the life cycle, with an impact on the requirements of certain micronutrients with aging; specific RDAs for older adults now have been developed. The mean vitamin B12 status of most populations, for example, declines significantly with older age, in large part because of the high prevalence of atrophic gastritis and its resultant impairment of protein-bound vitamin B12 absorption.29 Approximately 10% to 15% of the older ambulatory population is thought to have significant vitamin B12 depletion because of this phenomenon, and neuropathic degeneration may occur in older individuals whose plasma vitamin B12 levels are in the low-normal range (150 to 300 pg/mL), even in the absence of hematologic manifestations. For this reason, the use of sensitive indicators of cellular depletion of vitamin B12, such as serum methylmalonic acid levels in conjunction with serum levels of vitamin B12, now are recommended for diagnosis.30 Some experts also suggest that older adults should consume a portion of their vitamin B12 requirement in the crystalline form (i.e., as a supplement) rather than relying only on the naturally occurring protein-bound forms found in food.31 Older adults also require greater quantities of vitamins B6 and D and calcium to maintain health compared with younger adults, and these requirements are reflected in the new RDAs (see Tables 4-10 and 4-11).
Malabsorption and Maldigestion
Conditions that produce fat malabsorption frequently are associated with selective deficiencies of the fat-soluble vitamins. The early stages of many vitamin deficiencies are not apparent clinically and therefore may go undetected until progression of the deficiency has resulted in significant morbidity. This can be disastrous in conditions such as spinocerebellar degeneration from vitamin E deficiency, which often is irreversible.32 Fat-soluble vitamin deficiencies are well-recognized complications of cystic fibrosis and congenital biliary atresia in which fat malabsorption often is overt, but monitoring also is necessary in conditions associated with more subtle fat malabsorption, such as the latter stages of chronic cholestatic liver disease.33,34
Restitution of vitamin deficiencies sometimes can be difficult when severe fat malabsorption is present and initial correction may require parenteral administration. In severe fat malabsorption, chemically modified forms of vitamins D and E that largely bypass the need for the lipophilic phase of intestinal absorption are commercially available for oral use and can be helpful. The polyethylene glycol succinate form of vitamin E (Nutr-E-Sol) is very effective in patients with severe fat malabsorption who cannot absorb conventional alpha-tocopherol.35 Similarly, hydroxylated forms of vitamin D (1-hydroxyvitamin D [Hectorol] and 1,25-dihydroxyvitamin D [Rocaltrol]) can be used in patients resistant to the more conventional forms of vitamin D. Intermittent monitoring of serum calcium levels is indicated in the first few weeks of therapy when hydroxylated forms of vitamin D are administered because they are considerably more potent than vitamin D2 or D3 and risk of vitamin D toxicity exists. In contrast, water-miscible preparations of fat-soluble vitamins, in which a conventional form of vitamin A or E is dissolved in polysorbate 80 (e.g., Aquasol-E, Aquasol-A), have not been proven to improve overall absorption. At the time of this writing, Aquasol-A is no longer available as an oral supplement.
Maldigestion usually results from chronic pancreatic insufficiency, which if untreated frequently causes fat malabsorption and deficiencies of fat-soluble vitamins. Vitamin B12 malabsorption also can be demonstrated in this setting, but clinical vitamin B12 deficiency is rare unless other conditions known to diminish its absorption also are present, such as atrophic gastritis29 or chronic administration of proton pump inhibitors (PPIs).36 Whether the long-term administration of PPIs alone warrants occasional checks of vitamin B12 status is a matter of debate. Regardless, malabsorption of vitamin B12 from atrophic gastritis or with PPIs is confined to dietary sources of vitamin B12. Small supplemental doses of crystalline vitamin B12 are absorbed readily in both cases. Histamine H2 receptor antagonists also inhibit protein-bound vitamin B12 absorption, although the effect generally is believed to be less potent than with the PPIs.37
Many medications may adversely affect micronutrient status. The manner in which drug-nutrient interaction occurs varies; some of the more common mechanisms are described in Table 4-13. A comprehensive discussion of drug-nutrient interactions is beyond the scope of this chapter and the reader is referred to other references for a detailed discourse on this topic.38
Table 4-13 Interactions of Drugs on Micronutrient Status
| DRUG(S) | NUTRIENT | MECHANISM(S) |
|---|---|---|
| Dextroamphetamine, fenfluramine, levodopa | Potentially all micronutrients | Induces anorexia |
| Cholestyramine | Vitamin D, folate | Adsorbs nutrient, decreases absorption |
| PPIs | Vitamin B12 | Modest bacterial overgrowth, decreases gastric acid, impairs absorption |
| Sulfasalazine | Folate | Impairs absorption and inhibits folate-dependent enzymes |
| Isoniazid | Pyridoxine | Impairs uptake of vitamin B6 |
| NSAIDs | Iron | Gastrointestinal blood loss |
| Penicillamine | Zinc | Increases renal excretion |
NSAIDs, nonsteroidal anti-inflammatory drugs; PPIs, proton pump inhibitors.
From Goldman L, Ausiello D, Arend W, et al, editors. Cecil Textbook of Medicine. 22nd ed. Philadelphia: WB Saunders; 2004. With permission.
STARVATION
During the first 24 hours of fasting, the most readily available energy substrates (i.e., circulating glucose, FAs, and TGs, and liver and muscle glycogen) are used as fuel sources. The sum of energy provided by these stores in a 70-kg man, however, is only about 5000 kJ (1200 kcal) and therefore is less than a full day’s requirements. Hepatic glucose production and oxidation decrease, whereas whole-body lipolysis increases, and the latter provides additional FAs and ketone bodies.39 Oxidation of the FAs released from adipose tissue TGs accounts for approximately 65% of energy consumed during the first 24 hours of fasting.
During the first several days of starvation, obligate glucose-requiring tissues such as the brain and blood cells, which collectively account for about 20% of total energy consumption, can use only glycolytic pathways to obtain energy. Because FAs cannot be converted to carbohydrate, these glycolytic tissues must use glucose or substrates that can be converted to glucose. Glucogenic amino acids derived from skeletal muscle (chiefly alanine and glutamine) are a major source of substrate for this purpose. Approximately 15% of the REE is provided by oxidation of protein.40 The relative contribution of gluconeogenesis to hepatic glucose production increases as the rate of hepatic glycogenolysis declines because the latter process becomes redundant; after 24 hours of fasting, only 15% of liver glycogen stores remain.
During short-term starvation (1 to 14 days), several adaptive responses appear that lessen the loss of lean mass. A decline in levels of plasma insulin, an increase in plasma epinephrine levels, and an increase in lipolytic sensitivity to catecholamines stimulate adipose tissue lipolysis.41,42 The increase in FA delivery to the liver, in conjunction with an increase in the ratio of plasma glucagon-to-insulin concentrations, enhances the production of ketone bodies by the liver. A maximal rate of ketogenesis is reached by three days of starvation, and plasma ketone body concentration is increased 75-fold by seven days. In contrast to FAs, ketone bodies can cross the blood-brain barrier and provide most of the brain’s energy needs by seven days of starvation.43 The use of ketone bodies by the brain greatly diminishes glucose requirements and thus spares the need for muscle protein degradation to provide glucose precursors. If early protein breakdown rates were to continue throughout starvation, a potentially lethal amount of muscle protein would be catabolized in less than three weeks. Similarly, the heart, kidney, and skeletal muscle change their primary fuel substrate to FAs and ketone bodies. Other tissues such as bone marrow, renal medulla, and peripheral nerves switch from full oxidation of glucose to anaerobic glycolysis, resulting in increased production of pyruvate and lactate. The latter two compounds can be converted back to glucose in the liver using energy derived from fat oxidation via the Cori cycle, and the resulting glucose is available for systemic consumption. This enables energy stored as fat to be used for glucose synthesis.
During long-term starvation (14 to 60 days), maximal adaptation is reflected by a plateau in lipid, carbohydrate, and protein metabolism. The body relies almost entirely on adipose tissue for its fuel, providing more than 90% of daily energy requirements.44 Muscle protein breakdown decreases to less than 30 g/day, causing a marked decrease in urea nitrogen production and excretion. The decrease in osmotic load diminishes urine volume to 200 mL/day, thereby reducing fluid requirements. Total glucose production decreases to approximately 75 g/day, providing fuel for glycolytic tissues (40 g/day) and the brain (35 g/day) while maintaining a constant plasma glucose concentration. Energy expenditure decreases by 20% to 25% at 30 days of fasting and remains relatively constant thereafter, despite continued starvation.
The metabolic response to short- and long-term starvation differs somewhat between lean and obese persons. Obesity is associated with a blunted increase in lipolysis and decrease in glucose production compared with that in lean persons.45,46 In addition, protein breakdown and nitrogen losses are less in obese persons, thereby helping conserve muscle protein.47
The events that mark the terminal phase of starvation have been studied chiefly in laboratory animals. Body fat mass, muscle protein, and the sizes of most organs are markedly decreased. The weight and protein content of the brain, however, remain relatively stable. During this final phase of starvation, body fat stores reach a critical level, energy derived from body fat decreases, and muscle protein catabolism is accelerated. Death commonly occurs when there is a 30% to 50% loss of skeletal muscle protein.48 In humans, it has been proposed that there are certain thresholds beyond which lethality is inevitable—depletion of total body protein between 30% and 50% and of fat stores between 70% and 95%, or reduction of BMI below 13 kg/m2 for men and 11 kg/m2 for women.49,50
MALNUTRITION
PROTEIN-ENERGY MALNUTRITION
Illness or injury may directly interfere with nutrient assimilation; for example, extensive ileal disease or resection may directly produce fat malabsorption and a caloric deficit. The most common causes of secondary PEM, however, are the remarkable increases in protein catabolism and energy expenditure that occur as a result of a systemic inflammatory response. REE may increase as much as 80% above basal levels in a manner roughly proportional to the magnitude of the inflammatory response, which in turn is roughly proportional to the severity and acuity of the illness. Thus, for example, REE in patients with extensive second- and third-degree burns (the prototype for maximal physiologic stress) may approach twice normal; with sepsis, REE is about 1.5 times normal; and with a localized infection or fracture of a long bone, REE is 25% above normal.5 Such stress factors can be used to construct a formula for predicting the caloric needs of ill individuals (see Table 4-5).
Protein catabolism during illness or injury also increases in proportion to the severity and acuity of the insult, and therefore parallels the increase in energy consumption. The magnitude of increase in protein catabolism, however, is proportionately greater than that observed with energy consumption, such that urinary urea N losses, which reflect the degree of protein catabolism in acute illness, are about 2.5 times the basal level with maximal stress.5 This increase in catabolism results in a net loss of protein because the rate of synthesis usually does not rise in concert with the rise in catabolism.51 No known storage form of protein exists in the body and, therefore, any net loss of protein represents a loss of functionally active tissue. A healthy adult typically loses about 12 g N in the urine/day, and excretion may increase to as much as 30 g/day during critical illness. Because 1 g of urinary N represents the catabolism of approximately 30 g of lean mass, it follows that severe illness may produce a daily loss of up to ~0.5 kg of lean mass as a result of excess protein catabolism. Most of this loss comes from the skeletal muscle, where the efflux of amino acids increases two- to sixfold in critically ill patients.52
Mobilization of amino acids from skeletal muscle appears to be an adaptive response. Once liberated, these amino acids, in part, are deaminated and used for gluconeogenesis; they also are taken up by the liver and other visceral organs. The proteolysis of muscle under stress thus enables the body to shift amino acids from the skeletal muscle (the somatic protein compartment) to the visceral organs (the visceral protein compartment), the functions of which are more critical for immediate survival during illness. Nevertheless, with sustained stress, the limitations of this adaptive response become evident, and even the visceral protein compartment sustains a contraction in mass.44
Primary versus Secondary Protein-Energy Malnutrition: A Body Compartment Perspective
The type of tissue lost as malnutrition evolves is critical in determining the pathologic ramifications of weight loss. Over 95% of energy expenditure resides in the lean body mass, which therefore contains the bulk of metabolism that sustains homeostasis. It is the maintenance of this body compartment that is most critical for health. Lean body mass can be subdivided further into somatic and visceral protein compartments, blood and bone cells, and extracellular lean mass, such as plasma and bone matrix (Fig. 4-1). In total or semistarvation in otherwise healthy individuals, adipose tissue predominates as a primary energy source; thus, fat mass contracts to a much greater degree proportional to the loss of lean mass.44 Alterations in metabolism from injury or illness, however, produce a proportionately greater loss of muscle mass so that it matches or exceeds the loss in fat mass.53,54 Although the lean mass that is lost in illness preferentially is from the somatic protein compartment, with sustained stress there also will be a significant contraction of the visceral protein compartment (Table 4-14). The metabolic forces associated with acute illness and injury are potent, and restoration of muscle mass is unlikely with nutritional support unless the underlying inflammatory condition is corrected. There is increasing interest in attenuating or reversing net catabolism with the use of exogenous anabolic agents in conjunction with nutrition, although to date it remains unclear whether the clinical benefits of using exogenous growth hormone and other anabolic agents in acute illness outweigh their potential side effects.55,56
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree