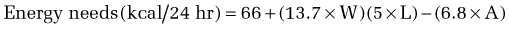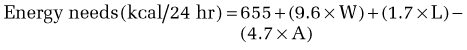CHAPTER 5 Nutrition in Gastrointestinal Diseases
NUTRITIONAL ASSESSMENT
Determining who is at risk for malnutrition is a complicated science. A proper nutritional assessment provides a mechanism whereby patients who may require nutritional support can be identified and also provides a gauge to monitor the effectiveness of such support (see Chapter 4).
MEDICAL HISTORY AND PHYSICAL EXAMINATION
Reading a patient’s chart, taking a history, and performing a physical examination allows a good understanding of a patient’s disease status and allows the diagnosis of some nutritional deficiencies. Inquiries regarding a patient’s usual body weight (UBW) versus ideal (IBW) or present body weight (PBW) should be noted at the initial patient encounter because these values have been shown in a number of studies to be predictors of morbidity and mortality.1,2 Although a simple tool, the most sensitive marker of nutritional risk is the percentage deviation from UBW over the past three to six months.
ANTHROPOMORPHIC MEASUREMENTS
Anthropomorphic measurements allow the estimation of body composition or body stores of energy using relatively simple and inexpensive equipment such as hand-held calipers (Fig. 5-1) and scales. Hand-held calipers allow measurement of a patient’s triceps skinfold (TSF), a marker of body fat stores, and midarm muscle circumference (MAMC), a marker of body protein stores. These measurements are compared with standardized tables to obtain percentages of normal values. Loss of body stores of protein (e.g., reduced MAMC) has been associated with poor patient outcomes.
Because of the obesity epidemic, emphasis has been placed on evaluating a patient’s weight and body habitus using the body mass index (BMI), which is defined as weight (in kilograms)/height (in meters squared). BMI values help categorize patients as underweight, normal weight, overweight, and obese (see Table 4-18).
BIOCHEMICAL MEASUREMENTS
The plasma proteins albumin, prealbumin, and transferrin are widely used to assess nutritional status. Albumin has been overrated as a predictor of protein malnutrition and, because it has a half-life of 21 days, it is a poor indicator of protein malnutrition. Infections, chronic medical conditions, liver disease, acute physiologic changes, and medications affect serum albumin levels through mechanisms not related to protein malnutrition.3 Low serum albumin levels (<3.5 g/dL) on hospital admission have been correlated with poor prognosis and poor surgical outcome.4
IMMUNOLOGIC TESTS
The serum total lymphocyte count has been correlated with changes in nutritional status, especially protein depletion.5 However, no prospective study has shown this to be a reliable marker of a patient’s nutritional status.
MUSCLE FUNCTION
An effective tool to evaluate a patient’s nutritional status over time is to measure his or her endurance or muscle strength. A practical method of assessing muscle strength involves hand grip strength,6 which is proportional to forearm lean muscle mass (Fig. 5-2). The reliability of this test is reduced for patients who are acutely ill or have hand or arm motor abnormalities.
GLOBAL ASSESSMENTS
To date, there is no single tool that is an accurate predictor of nutritional status. A variety of validated nutrition scoring systems, incorporating a number of features of the patient’s medical history and physical examination, has been developed to assess nutritional status. One of these, the subjective global assessment (SGA), is a comprehensive nutritional assessment tool that incorporates weight changes, dietary intake, functional capacity and preliminary medical diagnosis to categorize patients as well-nourished, moderately malnourished, or severely malnourished (see Table 4-24).7 The SGA has been validated in the oncology population.8 Other global nutritional assessment tools include the prognostic nutritional index (PNI), the instant nutritional assessment (INA), the Mini-Nutritional Assessment, the malnutrition universal screening tool, the nutritional risk score, and the nutritional risk index.9
CALORIC ASSESSMENT
Mathematical Equations
Calculation of energy requirements can be obtained through mathematical equations. Many different methods of estimating energy needs have been used over the years, including estimations based on body surface area, body weight, body height, and age of the patient. The most commonly used equation for calculating energy needs is the Harris-Benedict equation.10 The calculation is as follows:
where W = weight in kilograms, A = age, and L = height in centimeters.
This calculation of energy needs is multiplied by stress factors to arrive at a patient’s overall caloric needs (Table 5-1) Other caloric assessment formulas commonly used include the Ireton-Jones equation and the Mifflin-St. Jeor formula.11 Calculated energy needs, however, may over- or underestimate a patient’s true energy needs, especially in patients with complicated disease processes that can alter their metabolic rate.12 In these cases, direct measurements of overall energy needs may be more appropriate.
Table 5-1 Influence of Disease Severity (Physiologic Stress) on Resting Energy Expenditure (REE)
| DISEASE SEVERITY | REE (%) |
|---|---|
| Mild | 10 |
| Moderate | 25 |
| Severe | 50 |
Example: REE of 1500 kcal in a patient with moderate disease = (1500 kcal × 0.25) + 1500 kcal = 1875 kcal/day.
Indirect Calorimetry
Direct measurements of a patient’s energy needs can be performed using indirect calorimetry, which measures heat produced by oxidation. A ventilated hood is placed over the resting patient’s head and its oxygen and carbon dioxide content for two hours is analyzed. From this information, the resting energy expenditure (REE) is derived. True caloric needs are calculated by multiplying the REE by an activity or stress factor. In addition, a patient’s respiratory quotient (RQ) is derived. The RQ is equal to Vco2 (volume of CO2)/Vo2 (volume of O2). An RQ of approximately 0.7 or less is an indication of underfeeding whereas an RQ of approximately 1.0 or more is an indication of overfeeding.13 Indirect calorimetry plays a large role in obese or very malnourished patients, for whom determining caloric needs based on total body weight can be inaccurate.14 More recently, simple hand-held devices have been developed with an oxygen sensor for energy measurement. By breathing through the device for 10 minutes, the patient’s REE can be determined (Fig. 5-3).15 This tool has been shown to be as effective as the more expensive and laborious indirect calorimetry device in patients who can spontaneously breathe on their own volition, that is, those who are not on a ventilator.16
NUTRIENT SUBSTRATES
MACRONUTRIENTS
The macronutrients that fuel human metabolism include carbohydrates, fats, and proteins. The major source of energy in the human diet is carbohydrate, which constitutes almost half of the typical American diet. Carbohydrate is consumed as starch, sucrose, or lactose. Starch is made up of the polysaccharides amylopectin and amylose. Sucrose and lactose are disaccharides. Most digestion occurs in the duodenum and small intestine (see Chapter 100).17 Starch digestion begins in the mouth with the enzyme amylase and continues with the pancreatic enzyme alpha-amylase in the small intestine. Starch is broken down into oligosaccharides. The oligosaccharides and disaccharides are hydrolyzed by the small intestinal brush border to monosaccharides by glucoamylase, sucrase, and amylase. Glucose and galactose are absorbed across the small intestinal mucosa by active transport, whereas fructose moves across by facilitated diffusion.18
Dietary fat is primarily composed of triglycerides, which consist mainly of four long-chain fatty acids—palmitic, stearic, oleic, and linoleic—with smaller amounts of linolenic acid and medium-chain triglycerides (MCTs). Linoleic and linolenic acids are essential fatty acids; that is, they cannot be synthesized from nondietary sources. Essential fatty acid deficiency can result in a clinical syndrome consisting primarily of a scaly erythematous rash.19 Clinically, this may be seen in patients completely dependent on PN for their nutrient intake who receive no fat in their daily PN solution over a period of one to two weeks. Triglycerides are hydrolyzed to free fatty acids and beta-monoglycerides by pancreatic lipase and colipase.20 Because fats are insoluble in water, their digestion requires a unique environment involving an emulsification process whereby bile salts enhance their absorption (see Chapter 100). In water, bile salts form a micelle with a hydrophobic core and a hydrophilic periphery. Micellar contents diffuse across the water layer, intestinal mucosa, and cell membrane and enter the cell. Here they are reesterified to triglycerides and linked to an apoprotein to form a chylomicron.
Protein and amino acid metabolism are essential to provide the building blocks necessary to create a variety of body proteins and nitrogen-containing compounds. Proteins are composed of amino acids joined by peptide bonds. There are 22 amino acids, eight of which are essential: lysine, threonine, leucine, isoleucine, valine, methionine, phenylalanine, and tryptophan; two others, histidine and arginine, are essential for infants and growing children. Dietary proteins are partially digested by pepsin in the stomach to form polypeptides, but most protein digestion is performed in the duodenum and upper jejunum by pancreatic proteases, including trypsin, chymotrypsin, carboxypeptidase, and elastase (see Chapter 100).21 Peptides are further hydrolyzed in the small intestine by aminopeptidases, enzymes found in the brush border of the small intestine, to free amino acids and very small peptides. Large peptides must be hydrolyzed by brush border enzymes for absorption to occur, whereas dipeptides and tripeptides can move intact into mucosal cells.22
MACROMINERALS
Minerals account for only 4% of total body weight, yet they serve as essential cofactors, help maintain fluid osmotic pressures, and provide the proper environment for many chemical reactions (see Chapter 4).
Calcium
This is the most abundant cation in the human body. Bone and teeth contain about 99% of total body calcium. It is ingested in the form of an insoluble salt and must be released from its salt form to its ionized form for absorption (see Chapter 100).23 Calcium absorption occurs along the length of the small intestine and is vitamin D–dependent. In periods of restricted calcium intake, the colon may become more involved in calcium homeostasis by increasing its absorption. In periods of low serum calcium levels, renal excretion of calcium is reduced and bone calcium stores are released. Magnesium and calcium compete with one another for absorption. Unabsorbed dietary fat can interfere with calcium absorption by the formation of soaps in the intestine, which are excreted in the feces. The recommended daily intake of calcium is 800 mg/day for adults and 1200 mg/day for growing children. Calcium requirements are higher for pregnant women and older adults. In addition to bone mineralization, calcium also is important in blood clotting, muscle contraction, and the secretory activity of most endocrine and exocrine cells. Hypocalcemia may result in tetany, paresthesias, hyperreflexia, seizures, and mental status changes. Chronic calcium deficiency will result in rickets in children and osteomalacia in adults.
Magnesium
This is the second most abundant intracellular cation. Approximately 60% of magnesium is located in bone.24 Skeletal muscle also serves as another large source of magnesium storage. Magnesium absorption increases when magnesium intake is low. Vitamin D may affect absorption, although this relationship is not clear. Patients on a low-protein diet also can have difficulty with magnesium absorption. The recommended daily intake of magnesium is 300 to 500 mg/day. Magnesium is important in providing stability to the structure of ATP and is involved in numerous other enzyme systems. Magnesium deficiency can result in tetany, ataxia, myoclonus, coma, psychosis, cardiac dysrhythmias, and hypotension. Severe hypomagnesemia may be seen in patients with refeeding syndrome.
MICRONUTRIENTS
Essential micronutrients are present in minute or trace amounts in the body, sometimes in quantities less than 100 µg (see Chapter 4). Although trace elements are present in very small amounts, they often have dramatic effects; deficiencies are more common than toxicity. Deficiencies can result from reduced intake, decreased bioavailability, decreased transport proteins, excess excretion, or as the result of certain disease states. Many of these deficiencies develop in patients who are on long-term PN or who are severely malnourished. Assessment of trace element deficiency is extremely difficult (Table 5-2) because serum levels may not accurately reflect body stores. Therefore, clinicians may have to depend largely on physical signs and symptoms to detect micronutrient deficiency.
Table 5-2 Daily Trace Element Requirements
| TRACE ELEMENT | Requirement | |
|---|---|---|
| ENTERAL | PARENTERAL | |
| Chromium | 30 µg | 10-15 µg |
| Copper | 0.9 mg | 0.3-0.5 mg |
| Fluoride | 4 mg | Not well defined |
| Iodine | 150 µg | Not well defined |
| Iron | 18 mg | Not routinely added |
| Manganese | 2.3 mg | 60-100 µg |
| Molybdenum | 45 µg | Not routinely added |
| Selenium | 55 µg | 20-60 µg |
| Zinc | 11 mg | 2.5-5 mg |
From American Society for Parenteral and Enteral Nutrition. Guidelines for the use of parenteral and enteral nutrition in adults and pediatric patients. J Parenter Enteral Nutr 2002; 26:29SA-33SA.
Chromium
This is important in protein, carbohydrate, and lipid metabolism by serving as an important cofactor in enzymatic breakdown. It is crucial for the synthesis of glucose tolerance factor, a cofactor in insulin action.25 Chromium deficiency is manifested by glucose intolerance. The daily requirement is 50 to 100 µg/day.
Copper
This is important for normal body iron uptake. A microcytic hypochromic anemia can be seen with copper deficiency,26 a result of shortened red blood cell lifespan. Copper plays a major role in taste sensation and is important in reducing the potential injurious effects of free radicals. Copper is excreted in bile and should be replaced in those with external biliary drains or excessive diarrhea. The daily requirement is approximately 1.5 to 3 µg/day.
Zinc
This is an important component of many enzymes. It is involved in protein and lipid synthesis, and insulin activity. Approximately 25% of ingested zinc is absorbed daily in the duodenum and proximal jejunum. Excretion is through the biliary tract, skin, and feces. Zinc deficiency can result in a characteristic skin rash (acrodermatitis), poor wound healing, impaired taste, glucose intolerance, alopecia, depression, and diarrhea. Because body copper levels can be suppressed by zinc loading, zinc has been evaluated for the treatment of early Wilson disease.27 The daily requirement is approximately 10 to 15 mg/day.
VITAMINS
Vitamins (see Chapters 4, 100) are essential micronutrients involved in basic body functions such as growth, tissue maintenance, and metabolism. They are broadly classified as water-soluble or fat-soluble. Absorption of fat-soluble vitamins requires absorption and transport of lipids. Water-soluble vitamins, except vitamin C, are part of a B-complex group (Table 5-3).
Table 5-3 Daily Reference Intakes* and Sites of Absorption of Vitamins
| VITAMIN | RECOMMENDED DIETARY ALLOWANCE* | ABSORPTION SITES |
|---|---|---|
| C | 75-90 mg | Distal small intestine |
| B1 (thiamine) | 1.1-1.2 mg | Jejunum |
| Riboflavin | 1.1-1.3 mg | Proximal small intestine |
| Niacin | 14-16 mg | Stomach, small intestine |
| Pantothenic acid | 5 mg | Jejunum |
| Biotin | 30 µg | Unknown |
| Folic acid | 400 µg | Jejunum |
| B12 | 2-4 µg | Distal ileum |
| B6 | 1.3-1.7 µg | Jejunum |
| A† | 700-900 retinol equivalents | Proximal small intestine |
| D | 200-600 IU | Duodenum, terminal ileum |
| E | 15 mg/day | Mid–small intestine |
| K | 90-120 µg/day | Jejunum, ileum, colon |
* Daily Reference Intakes (DRI) were established by the Institute of Medicine between 1997-2001. They are quantitative estimates of nutrient intakes to be used for planning and assessing diets for healthy people. The DRIs include both recommended intakes and tolerable upper intake levels. The RDAs (Recommended Dietary Allowances) are a component of the DRIs and are defined as the daily intake of a nutrient considered sufficient to meet the requirements of 97% to 98% of adults.
† A retinol equivalent is 3.3 IU of vitamin A; 1 retinol equivalent = 6 µg β-carotene or 1 µg retinol.
Water-Soluble Vitamins
Niacin
This is found in animal foods such as beef, pork, and chicken and in cereal grains, especially wheat, rice, and bran. Niacin is hydrolyzed to niacinamide in the mucosal cells of the small intestine. It is important for the formation of the nucleotides nicotinamide adenine dinucleotide, reduced form (NADH) and NADPH, compounds that serve in a number of electron transport systems. Niacin is used in the treatment of hypercholesterolemia, although the mechanism whereby it reduces cholesterol levels is unclear. Side effects of therapeutic doses of niacin include flushing, liver injury, elevated uric acid levels, and dermatologic problems.28 Niacin deficiency may result in a constellation of symptoms referred to as pellagra, which is characterized by glossitis, coarse, scaly erythematous skin, diarrhea, and mental confusion.
Folic Acid
This is present in many foods including leafy vegetables, fruit, and liver. Polyglutamate forms of folate must be hydrolyzed by jejunal mucosal enzymes prior to absorption.29 Folate serves as a carbon donor in a number of synthetic reactions. The maturation of red blood cells and other short-lived cells is folate-dependent. Folate deficiency results in a macrocytic anemia because of decreased deoxyribonucleic acid (DNA) synthesis. Excessive folate ingestion may produce malaise, insomnia, and gastrointestinal distress.
Fat-Soluble Vitamins
Vitamin K
This is necessary for the synthesis of four of the 13 factors required for blood coagulation (II, VII, IX, and X). It also is important in the synthesis of four proteins involved in coagulation (C, Z, S, and M). It is found in plant and animal foods and is absorbed from the jejunum, ileum, and colon by an energy-dependent process or diffusion. Of the total ingested vitamin K, 8% is absorbed and its half-life is short, two to three hours. Bacteria in the digestive tract also can synthesize vitamin K. After liver conjugation, the excretion of vitamin K occurs in the bile, feces, and urine. The normal diet contains 300 to 500 mg of vitamin K/day; thus, deficiencies are rare, but can occur in patients with severe malnutrition, fat malabsorption, pancreatic insufficiency, cholestasis, or severe liver disease, or in patients receiving antibiotics. Toxicity is very rare and usually reported only in infants.30
NUTRITION IN SPECIFIC DISEASE STATES
INTESTINAL FAILURE (SHORT BOWEL SYNDROME)
Intestinal failure or short bowel syndrome results from loss or disease of the intestine, or both, to an extent that precludes adequate digestion and absorption (see Chapter 103); Crohn’s disease (see Chapter 111), intestinal trauma, and intestinal infarction (see Chapter 114) are the most common causes. The patient often presents with weight loss, diarrhea, dehydration, and weakness. Following extensive resection of the small intestine, intestinal rehabilitation (goal of resuming oral nutrition) of the remaining small intestine is more likely to be successful if the colon has been preserved and the ileocecal valve is maintained.31 The nutritional management of short bowel syndrome depends on the amount and location of small bowel removed. Initially, proton pump inhibitors are used to reduce gastric hypersecretion and anticholinergic agents are used to slow intestinal transit. Parenteral nutrition is prescribed to meet nutritional needs. Oral feedings are gradually started and the volume of PN is reduced as oral feedings are tolerated. If the patient has had a partial ileal resection and has an intact colon, cholestyramine can be used to reduce bile salt–induced diarrhea. In patients with a small amount of ileum remaining and an intact colon, however, the use of cholestyramine can increase diarrhea by creating a relative bile salt deficiency. Vitamin B12 should be given monthly. In patients with significant small bowel resections (80 to 100 cm remaining), a trial of a small-peptide, low-fat, enteral formula should be attempted to reduce the amount of PN the patient requires. Later, a polymeric enteral formula can be substituted. Patients with less than 80 cm of small intestine remaining and no colon often are PN-dependent. The use of somatostatin to reduce intestinal secretions and slow transit time remains controversial.32 Anticholinergic therapy should be initiated. Patients may require larger doses of anticholinergics than usually are recommended, because absorption of the oral medication is limited.
The use of growth hormone, glutamine, and a rice-based diet in an attempt to cause small bowel mucosal hypertrophy and better absorption is controversial. Early data have suggested very significant improvements in small intestinal absorptive function,33 not confirmed by subsequent studies.34 The use of a glycoprotein (GL-2) also has been postulated as a small intestine mucosal stimulator for improved absorption. A recent prospective evaluation of its efficacy noted a statistically significant reduction in PN use35; the effectiveness of this therapy is still being evaluated in additional studies.
PANCREATITIS
Nutritional support is imperative for patients with severe acute pancreatitis or relapsing chronic pancreatitis (see Chapters 58 and 59). Early PN appears to be associated with a reduction in the complications and mortality associated with acute pancreatitis compared with maintaining the patient on an NPO regimen.36 However, central line catheter sepsis rates are high and hyperglycemia is common. Enteral nutrition also has been used in patients with pancreatitis in contrast to previous beliefs that complete bowel rest was required. It appears that intrajejunal feedings are safe and well tolerated.37 A standard, fat-containing, polymeric enteral formula can be used.38 Randomized, prospective trials have shown a reduction in overall patient complications, hospital length of stay, and total hospital charges compared with the use of PN.39,40 The use of jejunal feeding in patients with chronic pancreatitis has been described to improve weight and reduce abdominal pain associated with eating.41 Gastric feedings also have been used successfully in patients with severe acute pancreatitis,42 but are still a topic of investigation.
CROHN’S DISEASE
Crohn’s disease (see Chapter 111) is sometimes associated with malnutrition.43 These patients often are hypermetabolic and may have anorexia because of nausea and abdominal pain. Deficiencies of magnesium, selenium, potassium, and zinc are common in inflammatory bowel disease (IBD) as a result of losses in diarrheal fluids and through fistula tracts.44 Dietary therapy in IBD always has been considered important, but no specific diet can be recommended. Fat restriction may be important in patients with ileal disease or those who have undergone an ileal resection. The use of EN can be an important component of IBD therapy for patients who cannot eat. Enteral nutrition has not proven superior to PN in inducing remissions in IBD,45 although it is less costly and associated with fewer complications. Enteral nutrition alone has not proven superior to drug therapy for the treatment of Crohn’s disease.46 The use of PN in IBD should be restricted to patients who have not responded to conservative medical therapy (EN and medications) or in whom EN cannot be delivered.
LIVER DISEASE
Nutritional deficiencies are common among patients with liver disease, mainly from decreased dietary intake, but also as a result of altered metabolism, decreased nutrient storage, and increased nutrient requirements (see Chapters 72, 92, and 93). Decreased nutrient intake is secondary to anorexia and nausea and is more common in patients with cirrhosis.47 Decreased bile salt production results in an intolerance to high-fat foods and the development of fat-soluble vitamin malabsorption. In addition, hypoalbuminemia results in edema of the mucosa of the small intestine, further compromising nutrient absorption. Depletion of muscle mass occurs secondary to a lack of adequate glucose stores and a dependency on protein stores for energy. Normal serum amino acid concentrations are altered, with a rise in aromatic amino acids (tyrosine, phenylalanine, and methionine) and a fall in branched-chain amino acids (valine, leucine, and isoleucine). The aromatic amino acids normally are removed by the liver; it is postulated that the rise in aromatic amino acids precipitates hepatic encephalopathy because they act as false neurotransmitters. Moreover, branched-chain amino acids are used preferentially as a protein source by patients in liver failure because they require minimal liver catabolism. Unfortunately, studies have failed to demonstrate improved outcomes in liver-failure patients fed a branched-chain amino acid–fortified diet or enteral solution.48 There is a general tendency to limit protein intake in patients with cirrhosis to prevent encephalopathy; however, these patients have an increased protein demand and further limiting their protein intake will only accelerate protein calorie malnutrition. It is preferable to feed patients according to their protein needs and treat encephalopathy with medications as it develops. Parenteral nutrition should be used with caution in patients with liver failure, because immune dysfunction places these patients at increased risk for catheter sepsis. In addition, the lack of liver glycogen stores can lead to episodes of hypoglycemia when patients are rapidly tapered off PN. Nutritional support prior to liver transplantation has been shown to improve patient outcome, especially in patients who are significantly malnourished prior to the transplantation.49
DIVERTICULAR DISEASE
Patients with diverticular disease (see Chapter 117) often are provided with incorrect nutritional information. They are told to avoid nuts or foods that contain seeds because of fear that the hard small particles may lodge in diverticula and precipitate diverticulitis. There are no clinical data to support this concept,50 and most data suggest that a high-fiber diet will reduce the occurrence of symptomatic diverticular disease.51 Patients hospitalized for complicated diverticular disease can remain symptom-free on a high-fiber diet.52 Fiber intake should be at least 25 g/day and should be provided as insoluble fiber, such as that contained in wheat bran, bran muffins, and fiber-based cereals. The use of probiotics has had some success in the treatment of and prevention of diverticulitis, although more work needs to be done in this area.53
DUMPING SYNDROME
Dumping syndrome is common after partial gastrectomy and vagotomy. Hypertonic gastric contents empty rapidly into the small intestine, and consequently up to 25% of plasma volume suddenly may be transferred to the small intestine.54 Nausea, cramping, diaphoresis, and palpitations develop. Nutritional therapy for dumping syndrome aims to deliver a lower osmolarity solution to the small intestine by the frequent ingestion of small meals containing fat, protein, and complex carbohydrates, but limited in simple sugars. Fluid intake should be restricted and separated from solid food intake to avoid rapid gastric transit. High pectin-containing foods (bananas, oranges) will slow gastric output.
CELIAC DISEASE
Small intestinal mucosal injury and consequent malabsorption in celiac disease (see Chapter 104) occur when a susceptible patient ingests gluten-containing foods such as wheat, barley, rye, or possibly oats. Patients, especially younger ones, present with classic signs of malabsorption, including diarrhea, cramping, and marked weight loss and often develop folate, iron, and fat-soluble vitamin deficiencies. The primary treatment is a gluten-free diet. Wheat starch free of gliadin forms the basis for most bread in a gluten-free diet. Corn, rice, and buckwheat are allowed. Most patients will improve with dietary management. The vast majority of commercially available EN products, if required, are gluten-free.
CANCER
Protein-calorie malnutrition is a common problem in cancer patients. Cancer cachexia is the consequence of multiple metabolic abnormalities induced by the tumor. Appetite stimulation has been used successfully in cancer patients with mild malnutrition.55 The routine use of aggressive nutritional support in all patients receiving chemotherapy and radiation is controversial. Prospective randomized studies have failed to show improved tolerance to chemotherapy with the use of nutritional support,56 and PN also has failed to show an improvement in morbidity from radiation therapy. Parenteral nutrition has been shown to be beneficial for patients with gastrointestinal obstruction from primary or metastatic tumors.57 Parenteral nutrition also has been found to be beneficial in patients following bone marrow transplantation who have developed severe gastrointestinal mucositis.58 Enteral nutrition has been used successfully in patients with head and neck cancer to prevent weight loss, reduce hospitalizations, and reduce interruptions in chemotherapy and radiotherapy. In summary, the use of nutritional support in the cancer patient should be restricted to those patients with a reasonable life expectancy who are likely to be unable to maintain their nutritional needs for a prolonged period. It is in these patients that an improved quality of life may occur.
OBESITY
Obesity is a disease that has had explosive growth, simultaneously affecting many individuals in a community without regard to age, gender, or ethnic origin (see Chapter 6).59 Traditionally, the gastroenterologist has been involved in the endoscopic management of postbariatric surgical complications, including stomal stenosis, gastrointestinal bleeding, and fistulization (see Chapter 7). Previous endoscopic technologies used to treat obesity endoscopically, such as the gastric balloon, had limited exposure in the United States and were removed from the market because of associated complications, such as balloon deflation with migration and resultant small intestinal obstruction. Internationally, however, balloon endoscopic therapy continues, with a number of favorable outcomes reported.60,61 Patients with obesity-related gastrointestinal disease, such as gastroesophageal reflux disease, always have been a staple of the gastroenterologist’s practice. Today, gastroenterologists find themselves on the front line with other physicians attempting to combat obesity and its associated complications, including assessment of patients for risk, identification of those who may benefit from weight loss therapy, and determination of the appropriate weight loss intervention, including management of therapy-associated complications.
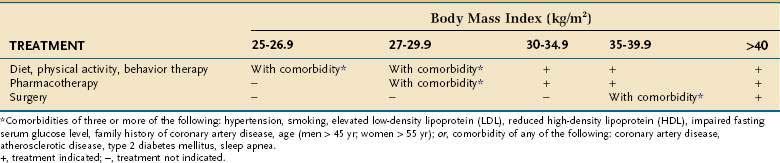
Assessing patients with obesity generally takes into consideration the BMI, waist circumference, and comorbid disease states. A person with a BMI more than 30 kg/m2 is considered obese. Patients who are overweight (BMI, 25 to 30 kg/m2) with comorbid diseases should be offered dietary management, exercise, and behavioral therapy. Patients with a BMI more than 30 kg/m2 also should be offered medical management (Table 5-4). If these patients have comorbid diseases, they also should be offered pharmacotherapy (weight loss medications). Patients with a BMI more than 35 kg/m2 should be offered medical management and pharmacotherapy. Surgical therapy is recommended for patients with a BMI more than 35 kg/m2 with significant comorbid diseases and who have not responded to medical management and pharmacotherapy. Patients with a BMI more than 40 kg/m2 should be offered medical management, pharmacotherapy, and surgery.
An enlarged waist circumference also places a patient at higher medical disease risk, separate from comorbid diseases, and correlates well with total body fat.62 Men with a waist circumference more than 40 inches or women with a waist circumference more than 35 inches have a higher risk, similar to the presence of significant comorbid diseases in a man with a BMI more than 30 kg/m2. These patients would be offered pharmacotherapy in addition to medical management, similar to the management of a patient with a BMI more than 30 kg/m2 with significant comorbid disease.
Surgical management of obesity in the United States typically involves a Roux-en-Y gastric bypass, vertical banded gastroplasty, or gastric banding (see Fig. 7-1). In general, most weight is lost in the first year.63 Gastric bypass surgery has a reasonable five-year outcome for maintenance of successful weight loss, but there is an associated mortality (0.5% to 2%) and complications associated with surgical therapy, some of them very significant.64,65
NUTRITIONAL THERAPY
PARENTERAL NUTRITION
PN delivers a solution consisting of water, electrolytes, amino acids, carbohydrates, fats, proteins, vitamins, and trace elements. These compounds are mixed and delivered over a period of time, usually 12 to 24 hours. Table 5-5 details a typical CPN formula. A CPN solution is six times more concentrated than blood (1800 to 2400 mOsm/L) and generally consists of approximately 30 to 50 g of protein and 1000 to 1200 cal/L. Determination of caloric and protein needs is based on a prior nutritional assessment.66 Table 5-6 provides an approximation of a patient’s daily protein and calorie needs based on the severity of the disease processes. These needs could be used instead of more complicated formulas presented earlier in this chapter for determining calorie and protein requirements. Overall, daily water requirements are estimated at 20 to 30 mL/kg.
Table 5-5 Sample Central Parenteral Nutrition Order*
| COMPONENT | AMOUNT |
|---|---|
| Amino acids | 220 kcal/L (55 g protein) |
| Dextrose | 555 kcal/L (163 g carbohydrates) |
| Lipids | 400 kcal/L (40 g of lipids), total: 1175 kcal/L |
| Sodium | 70 mEq/L |
| Potassium | 35 mEq/L |
| Calcium | 5 mEq/L |
| Magnesium | 5 mEq/L |
| Phosphorus | 15 mmol/L |
| Chloride, acetate | To balance |
| Multivitamins (MVI-13) | Industry standard |
| Trace elements (MTE-5) | Industry standard |
| Drug additives/L (heparin, insulin, H2 blockers) | Individualized |
* Volume: 2000 mL (83 mL/hr over 24-hr infusion).
Table 5-6 Calorie and Protein Needs Based on Degree of Physiologic Stress
| PHYSIOLOGIC STRESS | CALORIE NEEDS (KCAL/KG/DAY) | PROTEIN NEEDS (G/KG/DAY) |
|---|---|---|
| Mild | 25-28 | 0.8-1.0 |