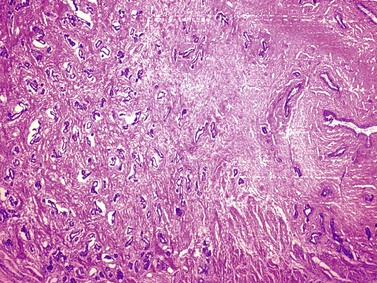
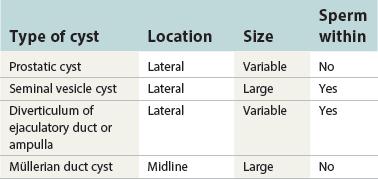
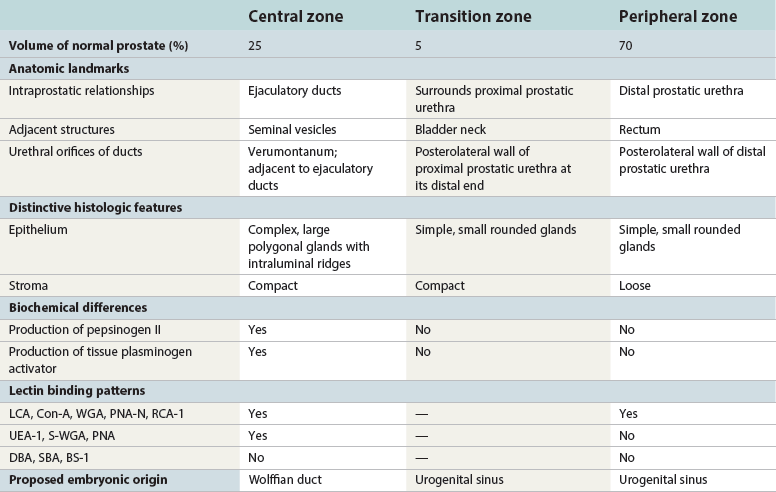
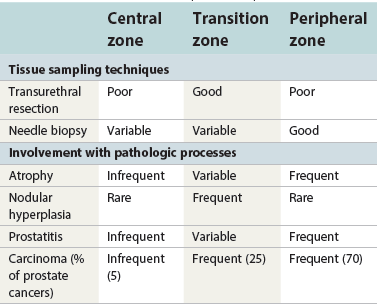
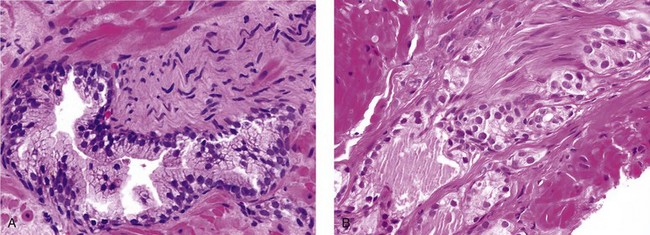
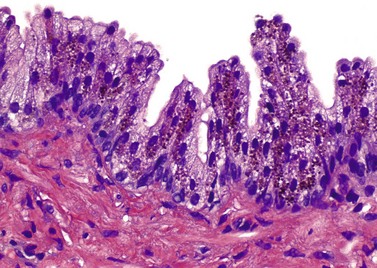
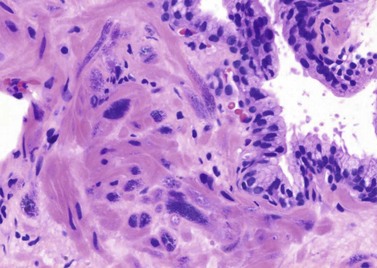
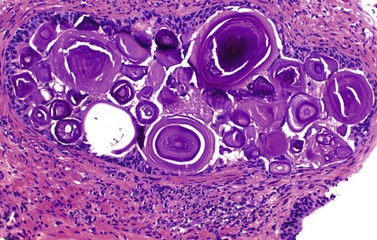
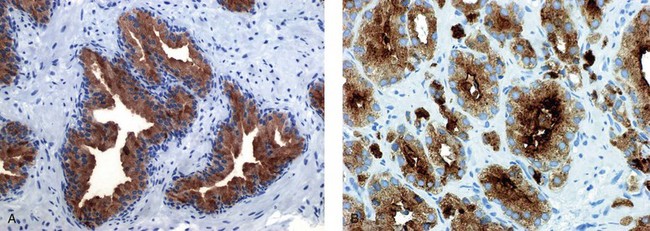
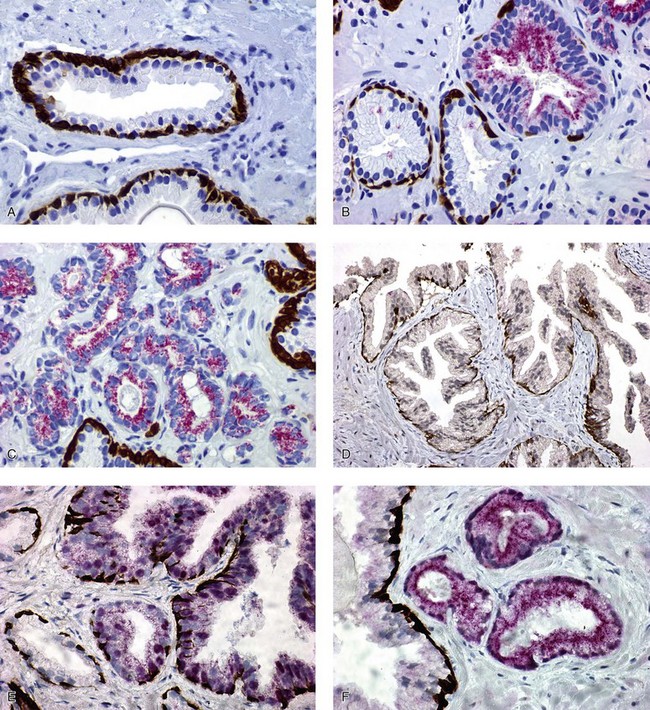
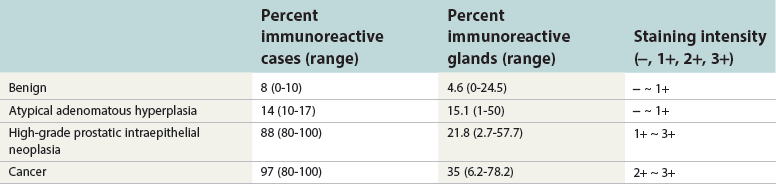
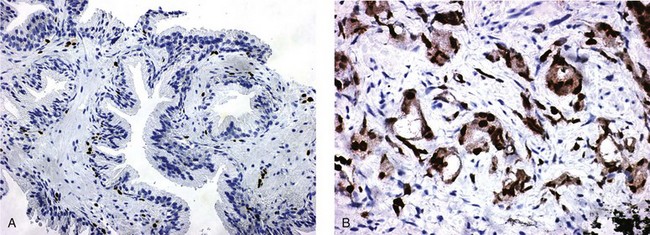
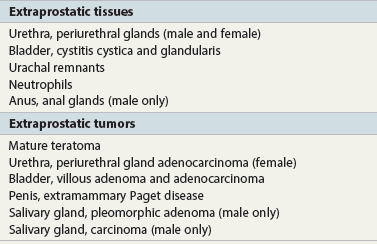
Chapter 8 Embryology and fetal-prepubertal history Anatomy and histology of the prostate Prostatic urethra and verumontanum Normal epithelium of the prostate Seminal vesicles and ejaculatory ducts Hyperplasia and nodular hyperplasia Usual acinar and stromal hyperplasia Postatrophic hyperplasia (postinflammatory hyperplasia; postsclerotic hyperplasia) Prostatic stromal hyperplasia with atypia Basal cell hyperplasia and basal cell proliferations Atypical adenomatous hyperplasia (atypical hyperplasia; adenosis) Verumontanum mucosal gland hyperplasia Benign nonneoplastic conditions The prostate is derived from the urogenital sinus.1 During the first 10 weeks of gestation, testosterone from the embryonic testes stimulates ingrowth of epithelial buds into urogenital sinus mesenchyme through a feedback loop.1 The mesenchyme induces the urogenital sinus epithelium to undergo ductal morphogenesis and differentiation that, in turn, signals the urogenital mesenchyme to differentiate into smooth muscle cells surrounding the epithelial ducts.1–3 Concurrently, the seminal vesicles, epididymis, vas deferens, and ejaculatory ducts develop from wolffian (mesonephric) ducts stimulated by fetal testosterone. At 31 to 36 weeks of gestation, the basic structure of the prostate is fully formed. In the fetal prostate, prostatic acini consist of tight aggregates of immature basal cells, often with squamous metaplasia of ducts and the urethra (Fig. 8-1). Immunohistochemical expression of estrogen receptor (ER) β is evident at 7 weeks throughout the urogenital epithelium, ejaculatory ducts, müllerian ducts, and stroma in all zones, and persists through at least 22 weeks.4 ER α is first detectable at 15 weeks of gestation, with minimal staining in the utricle. By 19 weeks, increased expression is present in the luminal cells of the ventral urogenital epithelium, basal cells of the dorsal urogenital epithelium, utricle, distal periurethral ducts, peripheral stroma, and posterior prostatic duct, and this expression becomes more intense in the following weeks. After birth, the size of the prostate remains stable until 10 to 12 years of age, but duct formation and solid epithelial outgrowth continue.5–8 During puberty, marked androgen-driven increase in gland size occurs. By age 20 years, the mean prostate weighs approximately 20 g and remains stable for up to 30 years.1,6,7 Maldevelopment of the prostate is rare and includes aplasia, hypoplasia, cystic change, and mesonephric remnants. Aplasia and hypoplasia are associated with androgen deficiency. Cystic change is uncommon and may be congenital or acquired, including utricular and müllerian duct cyst, ejaculatory duct cyst,9–11 vas deferens cyst, and seminal vesicle cyst; these cysts rarely produce clinical symptoms (Table 8-1).11–13 Cysts may be also associated with infertility secondary to ejaculatory duct obstruction.14,15 Mesonephric remnants in the prostate are an unusual mimic of adenocarcinoma16–20 that consists of a proliferation of benign acini arranged in lobules or showing infiltrative growth between smooth muscle bundles without stromal desmoplasia (discussed later).20 The surgical anatomy of the prostate is varied and complex, and it significantly influences continence, erectile function, and cancer control.21 The capsule of the prostate consists of an inner layer of smooth muscle and an outer covering of collagen, with marked variability in the relative amounts in different areas (Fig. 8-2). The collagenous fascia surrounding the prostate is multilayered and may fuse with the prostate capsule depending on location and interindividual variation.21 Fig. 8-2 Whole-mount prostate showing the capsule and neurovascular bundles (bottom right). Contrast with the contralateral region (bottom left) where the bundle has been spared and left within the patient. At the apex, the acinar elements become sparse, and the capsule becomes ill-defined, composed of a mixture of fibrous connective tissue, smooth muscle, and striated muscle. As a result, the prostatic capsule cannot be regarded as a well-defined anatomic structure with constant features.1,22 In biopsy and surgical specimens, the capsule at the apex and bladder base is difficult to identify; consequently, it is often not possible to determine the presence of extraprostatic extension of cancer at these sites. At the lateral aspect of the prostate, the lateral pelvic fascia and the prostatic capsule are separated by adipose tissue in 52% of cases, whereas they are adherent in the remainder.23 Denonvilliers fascia and the prostatic capsule are tightly adherent posteriorly in 97% of cases. A smooth transition from the prostatic capsule to the anterior fibromuscular stroma is invariably present, but the prostatic capsule is recognizable only anteriorly in 11% of cases. The lateral pelvic fascia connects and fuses with the anterior fibromuscular stroma and covers the outermost regions of the lateral and anterior surfaces in 85% of cases.23 Abundant adipose tissue is present around most the prostate and is a reliable marker of extraprostatic tissue in biopsy samples. In a series of radical prostatectomies, periprostatic adipose tissue was present on 48% of all prostatic surfaces examined.24 The distribution varied among the different surfaces of the prostate, with the anterior, posterior, right, and left surfaces showing 44%, 36%, 59%, and 57% adipose tissue, respectively. Nerve-sparing prostatectomy resulted in slightly less adipose tissue (46%) than did non–nerve-sparing procedures (54%). Intraprostatic fat is very rarely observed and, when present, consists of a small microscopic focus of a few adipocytes.25,26 For practical purposes, identification of fat in biopsy specimens is considered sampling of extraprostatic tissue. The urethra serves as a reference landmark for the study of prostatic anatomy (Fig. 8-3).27 A single 35-degree bend in the center of the prostatic urethra creates proximal and distal segments of nearly equal length. The verumontanum bulges from the posterior wall at the urethral bend and tapers distally to form the crista urethralis. Most prostatic ducts and the ejaculatory ducts empty into the urethra in this part of the middle and distal prostatic urethra, whereas the small periurethral glands of Littre have minute openings throughout the length of the urethra. Just proximal to the verumontanum is the utricle, a small, 0.5-cm long epithelium-lined cul-de-sac. More recent studies reveal that the utricle is derived from the urogenital sinus, in contrast to the previous belief that the utricle is a müllerian remnant.28,29 Careful microdissection study revealed three types of utricle based on the location of its pouch; in the most common type, the utricle projected out from between the two ejaculatory ducts.30 The site and shape of the utricular orifice were also diverse; this orifice was most commonly located on the distal three fourths of the prostatic urethra. Fig. 8-3 A, Sagittal diagram of the distal prostatic urethral segment (UD), proximal urethral segment (UP), and ejaculatory ducts (E), showing their relationships with a sagittal section of the anteromedial nonglandular tissues (bladder neck [bn], anterior fibromuscular stroma [fm], preprostatic sphincter [s], distal striated sphincter [ds]). These structures are shown in relation to a three-dimensional representation of the glandular prostate (central zone [CZ], peripheral zone [PZ], transition zone [TZ]). The coronal plane (C) and the oblique coronal plane (OC) are indicated by arrows. B, Oblique coronal section diagram of the prostate showing location of the PZ and TZ in relation to the UP, verumontanum (V), preprostatic sphincter [s], bn, and periurethral region with periurethral glands. The branching pattern of the prostatic ducts is indicated; medial TZ ducts penetrate into the sphincter. A circumferential sleeve of muscle surrounds the entire urethra. This muscular layer includes a proximal preprostatic smooth muscle sphincter that prevents retrograde ejaculation and a distal sphincter of striated and smooth muscle at the apex that is important in control of micturition.31 The prostate is composed of three zones: the peripheral zone, central zone, and transition zone (Tables 8-2 and 8-3).1,2,32,33 The peripheral zone contains approximately 70% of the volume of the prostate and is the most common site of prostatic intraepithelial neoplasia (PIN) and carcinoma. Peripheral zone acini are simple, round to oval, and set in a loose stroma of smooth muscle and collagen (Fig. 8-4). Digital rectal examination often includes a description of the left and right “lobes” based on palpation of the median furrow in the midline that divides the peripheral zone into left and right halves.1,2,32,33 Fig. 8-4 Normal peripheral zone (A), consisting of simple acini and a loose stroma of smooth muscle and collagen. The epithelium (B) is columnar, with small round basal nuclei and an inconspicuous flattened basal cell layer. The central zone is a cone-shaped area that includes the entire base of the prostate and encompasses the ejaculatory ducts; it comprises approximately 25% of the volume of the prostate. Central zone acini are large and complex, with intraluminal ridges, papillary infoldings, and occasional epithelial arches and cribriform glands mimicking PIN (Fig. 8-5).1,20,34 The ratio of epithelium to stroma is higher in the central zone than in the rest of the prostate, and the stroma is composed of compact interlacing smooth muscle bundles.1,32 Fig. 8-5 Normal central zone (A), consisting of large acini with complex intraluminal ridges, papillary infoldings, and epithelial arches set in a stroma of compact smooth muscle. The epithelium (B) varies from cuboidal to columnar. The transition zone contains the smallest volume of the normal prostate, approximately 5%, but it often enlarges together with the anterior fibromuscular stroma to massive size in benign prostatic hyperplasia (BPH) and dwarfs the remainder of the prostate. Transition zone glands tend to be simple, small, and round, similar to those in the peripheral zone, but they are embedded in a compact stroma that forms a distinctive boundary with the loose stroma of the peripheral zone (Fig. 8-6).1,32,35 The central zone and peripheral zone are often referred to together as the outer prostate or “nontransition zone,” whereas the transition zone and anterior fibromuscular stroma are often referred to as the inner prostate.32 Fig. 8-6 Normal transition zone (A), consisting of simple acini and a compact stroma. The epithelium (B) is cuboidal or low columnar with apical cytoplasmic blebs. Gene expression differs between the peripheral zone and the transition zone,36,37 and stromal-epithelial interactions may be responsible for the distinct zonal localization of diseases, especially cancer. For example, ERG and ETV-1 are upregulated in the glands of the peripheral zone compared with the transition zone; this finding may be important when considering that ERG and ETV-1 fusions account for 50% to 80% and 20% of prostate cancer occurrences, respectively.38 Stromal cells from the peripheral zone appear to have a greater capacity to induce development and progression of cancer than do stromal cells from the transition zone through growth factors regulated by sex hormones.39 The nerve supply of the prostate is furnished by paired neurovascular bundles that run along the posterolateral edge of the prostate from apex to base, situated between the fascial layers covering the prostate and seminal vesicles. These bundles consist of numerous nerve fibers superimposed on a scaffold of veins, arteries, and variable amounts of adipose tissue surrounding almost the entire lateral and posterior surfaces of the prostate. At the apex and the urethra, the neurovascular bundles have two divisions: cavernous nerves (continuation of the anterior and anterolateral fibers around the apex of the prostate, traveling toward the corpora cavernosa) and corpus spongiosum nerves (continuation of the posterolateral bundles that eventually reach the corpus spongiosum).40,41 Surgical sparing of these structures during radical prostatectomy may preserve sexual potency.42 Variation in size and shape of the prostate significantly influences the anatomy of the neurovascular bundles, the urethral sphincter, the dorsal vascular complex, and the pubovesical and puboprostatic ligaments.21 Autonomic ganglia are clustered near the neurovascular bundles, send out small nerve trunks that arborize over the surface of the prostate, penetrate the capsule, and branch to form an extensive network of nerve twigs within the prostate that are often in intimate contact with the walls of ducts and acini.43 Capsular ganglia are present in 52% of radical prostatectomy specimens, most frequently at the posterolateral aspect of the base. No obvious morphologic differences exist between capsular and periprostatic ganglia.44 The posterior capsule has significantly more nerve fibers than the anterior capsule, according to S100 protein staining.43 Caution is warranted in interpretation of perineural space invasion as an absolute criterion for the diagnosis of cancer because this feature can be seen rarely in benign glands (Fig. 8-7).20,45 Innervation of the peripheral zone is significantly greater than in the transition zone, according to an immunohistochemical survey using S100 protein, and that is, in turn, significantly more innervated than the prostate in BPH.43 The posterior capsule has significantly more nerve area than the anterior capsule. The greatest amount of neural tissue is present in the neurovascular bundles and seminal vesicles, with the lowest in the transition zone and in BPH. Innervation appears to decrease with age. The blood supply of the prostate is furnished by one of the branches of the internal iliac artery. In one report, the most frequent origin of prostatic arteries was the internal pudendal (56%), followed by the common gluteal-pudendal trunk (28%), the obturator (12%), and the inferior gluteal (4%).46 Anastomoses were noted with the termination of the internal pudendal artery in 24% of cases, with the contralateral arteries in 12%, and to the superior vesical artery in 8%. Prostatic and capsular arteries run along the lateral border of the prostate and serve as a visual landmark in the majority of cases for cavernosal nerve-sparing prostatectomy.47 Arterial embolization has provided good symptom control in patients with nodular hyperplasia.48 Lymphatics from the prostate drain mainly into the internal iliac lymph nodes, with lesser drainage into the external iliac and sacral lymph nodes.1 Antibodies directed against lymphatic vessel endothelial hyaluronan receptor (LYVE-1) revealed that lymphatic density was greater in BPH than in carcinoma, whereas microvessel density with anti-CD34 antibodies revealed the opposite.49 The epithelium of the prostate is composed of three principal cell types, according to light microscopy: secretory cells, basal cells, and neuroendocrine cells. An additional cell type, intermediate cells, can be convincingly demonstrated only by its unique immunophenotype (keratin phenotype intermediate between basal and luminal cells that coexpresses high levels of keratin 5 and keratin 18 [K5/18] and hepatocyte growth factor receptor c-MET).50 The secretory luminal cells are cuboidal to columnar, with small, round nuclei, punctate or inconspicuous nucleoli, finely granular chromatin, and pale to clear cytoplasm; they account for the bulk (73%) of the epithelium volume.1,4,32 Despite having the lowest proliferative activity, the terminally differentiated secretory cells produce prostate-specific antigen (PSA), prostatic acid phosphatase (PAP), androgen receptors, acidic mucin, and other secretory products. They also express high levels of keratins 8 and 18, but they lack keratins 5 and 14, as well as p63. The basal cells of the prostate form a flattened, attenuated layer of inconspicuous elongate cells at the periphery of the glands surmounting the basement membrane (Fig. 8-8).51 These cells possess the highest proliferative activity of the prostatic epithelium, albeit low, and are thought to act as stem or “reserve” cells that repopulate the secretory cell layer.50–53 Basal cells apparently retain the ability to undergo metaplasia, including squamous differentiation in the setting of infarction and myoepithelial differentiation in sclerosing adenosis. Epidermal growth factor receptors have been identified in basal cells but not in secretory cells, a finding suggesting that these cells play a role in growth regulation.54–56 Basal cells are selectively labeled with antibodies to high-molecular-weight keratins such as clone 34βE12 (keratin 903), a property that is exploited immunohistochemically in separating benign acinar processes such as atrophy, which retains a basal cell layer, from cancer, which lacks a basal cell layer (see Fig. 8-8). The nuclear protein p63 is another diagnostically useful basal cell marker that consistently decorates nuclei.57 Basal cell cocktail (the combination of 34βE12 and p63) increases the sensitivity of basal cell detection and reduces staining variability compared with either stain alone.26,58 Basal cells also express high levels of keratins 5 and 14, as well as p63, in contrast to secretory and intermediate cells. Basal cells contain little or no PSA, PAP, androgen receptors, keratins 8 or 18, or mucin. The normal prostatic epithelium frequently displays foci of basal cell proliferation that are too small to warrant the diagnosis of basal cell hyperplasia. Prostatic basal cells do not possess myoepithelial differentiation, unlike basal cells in the breast, salivary glands, pancreas, and other sites, probably because the massive smooth muscle stroma of the prostate propels secretions downstream without need for assistance from basal cells. Fig. 8-8 A, Normal prostatic acinus with prominent basal cell layer resulting from nuclear crowding and hyperchromasia. The basal cell layer is usually inconspicuous. B, Immunostain for high-molecular-weight keratin and p63 demonstrates a continuous circumferential layer of basal cells. Neuroendocrine cells are the least common cell type of the prostatic epithelium and are usually not identified in routine hematoxylin and eosin (H&E)–stained sections, except for rare cells with large eosinophilic granules.59–61 Although their function is unknown, neuroendocrine cells probably have an endocrine-paracrine regulatory role in growth and development, similar to neuroendocrine cells in other organs, and contain numerous neuropeptides that modulate cell growth and proliferation.60,62,63 Androgen deprivation therapy does not appear to influence the number or distribution of neuroendocrine cells in the normal or neoplastic prostate.64 These cells coexpress PSA64 and androgen receptors,65 a finding suggesting a common cell of origin for epithelial cells and neuroendocrine cells in the prostate. That neuroendocrine cells are most numerous near the verumontanum suggests a role in luminal constriction and dilatation. Serotonin and chromogranin are the best immunohistochemical markers of neuroendocrine cells in formalin-fixed sections of the prostate (Fig. 8-9).59,62,66 African American men have comparatively low prostatic neuroendocrine cell expression compared with other ethnic groups, and this may play a role in their increased risk of cancer.67 Androgen receptors appear to play a significant regulatory role in maintaining the balance between progenitor cells and luminal secretory cells. Androgen receptors are expressed at a low level in basal cells, but they play a suppressive role in proliferation of the CK5(+)/CK8(+) progenitor/intermediate cells and a positive (proliferative) role in the CK5(−)/CK8(+) luminal epithelial cells.68 Stem cell–derived clonal units found in the normal, atrophic, hyperplastic, and dysplastic (PIN) epithelium actively replenish the epithelium during aging.69 These deficient areas usually include the basal compartment, a finding indicating the basal layer as the location of the stem cell. Fig. 8-9 Neuroendocrine cells. A, When present in normal prostatic epithelium, these cells are infrequent and variable in shape (serotonin immunohistochemical stain). B, The cells in prostatic adenocarcinoma display dark cytoplasmic reaction product that fills the cytoplasm of scattered cells and obscures the nuclei (chromogranin immunohistochemical stain). Pigment is occasionally observed in the cytoplasm of the secretory epithelium, including lipofuscin and melanin.70,71 Lipofuscin granules are golden brown, gray brown, or blue by H&E staining (Fig. 8-10) and positive for Fontana-Masson, periodic acid–Schiff (PAS) with diastase, Congo red, Luxol fast blue, and oil red O stains, and they exhibit autofluorescence. Similar pigment may be also found in seminal vesicle and ejaculatory duct epithelium, high-grade PIN, and adenocarcinoma. Special attention should be given to avoid misinterpretation of pigmented acini adjacent to carcinoma as evidence of seminal vesicle invasion on needle biopsy. This pigment represents “wear and tear” or “old age” pigment resulting from endogenous cellular byproducts of the prostate epithelium.72 It is present in all zones of the prostate and is randomly distributed. Less commonly, melanin-like (Fontana-Masson–positive) pigment is found in scattered foci in the normal and hyperplastic prostatic epithelium and stroma (Fig. 8-11) (see the later discussion of melanosis).73 Intraluminal products of benign prostatic acini include mucin, degenerating epithelium, crystalloids, proteinaceous debris, prostasomes, corpora amylacea, calculi, and spermatozoa.74,75 Mucin is usually absent in benign acini. Histochemical staining demonstrates neutral mucins (PAS with diastase), whereas neoplastic acini often demonstrate neutral and acidic mucins (Alcian blue, pH 2.5 positive).76–79 However, acid mucin is not specific for prostate cancer because certain benign conditions also express it, including mucinous metaplasia, sclerosing adenosis, atypical adenomatous hyperplasia, and high-grade PIN. Mucins MUC1, MUC2, MUC4, MUC5AC, and MUC6 are not found in benign prostatic epithelium,80 although conflicting results have been found with MUC181,82 and MUC4.83 Positive staining for MUC6 in seminal vesicle epithelium may be useful for excluding prostatic origin.84 Corpora amylacea are luminal secretions present in up to 78% of benign prostatic acini,75 but they are observed only rarely in stromal smooth muscle cells85 and adenocarcinoma (0.4% of needle biopsies with cancer) (Figs. 8-12 and 8-13).75 They vary in size and shape, but most are round (Fig. 8-14). Color ranges from pink purple to orange, and the presence of concentric laminations is variable. Corpora amylacea are thought to be related to epithelial cell desquamation, degeneration, and inflammation. Ultrastructurally, they are composed of bundles of fibrils and occasional interspersed electron-dense areas.86 Biochemical analysis and x-ray diffraction revealed that the main constituent is sulfated glycosaminoglycans.87,88 Other constituents include lactoferrin, calprotectin, myeloperoxidase, and α-defensins, proteins contained in neutrophil granules, thus suggesting a role for acute inflammation in the pathogenesis of corpora amylacea.89 Proteinaceous secretions are a frequent nonspecific finding in benign and neoplastic acini (overall 8% incidence in one study, with 100% incidence in men >70 years of age),90 and they are considered the precursors of corpora amylacea and calculi.91 In an animal model, luminal secretions increased significantly in the presence of inflammation.92 Prostasomes are prostate-derived membranous vesicles that can be isolated from seminal plasma.93 They have been proposed to perform a variety of functions, including modulation of (immune) cell activity within the female reproductive tract and stimulation of sperm motility and capacitation.94 Calculi (microcalcifications), present in 31% to 100% of prostates, are typically found in central, large prostatic ducts (Fig. 8-15),95 composed predominantly of calcium phosphate in the form of hydroxyapatite.89 Suh and colleagues reported that calcifications in the prostate and ejaculatory system were common, found in 88.6% (264/298) of prostates, 58.1% (173/298) of seminal vesicles, and 17.1% (51/298) of ejaculatory ducts.96 The prostatic calcifications occurred mostly in benign glands and/or stroma of all zones and the verumontanum. Calcifications were more common in the transition zone than in other zones. They were often observed in association with inflammation, BPH, and basal cell hyperplasia.74,97 Whereas luminal microcalcifications are commonly observed in breast cancer, prostatic calculi are rarely seen in malignant acini. Interestingly, stromal microcalcifications are observed in approximately 3% of needle biopsy specimens, invariably in association with chronic inflammation.98 Calcific deposits of Mönckeberg medial calcinosis are occasionally observed in arteries and large arterioles in the periprostatic tissue. Calcifications were more prevalent in autopsy prostates from African Americans in Washington, DC than from Africans from West Africa, a finding perhaps reflecting different diets of the two population groups.99 A positive association was noted between prostatic calcification and age. Prostatic calcification is also common in patients with chronic pelvic pain syndrome and is associated with greater inflammation, bacterial colonization, and symptom duration.100 Intraprostatic spermatozoa can be identified with Berg stain; they are present in 26% of prostates at radical prostatectomy, including the peripheral zone (72%), central zone (22%), and transition zone (6%).101 They are frequently associated with inflammation and atrophy, including postatrophic hyperplasia. Small, 15- to 20-µm eosinophilic hyaline bodies, referred to as stromal hyaline bodies, are sometimes observed within the muscular wall of the seminal vesicles and vas deferens and stroma of the prostate.102 These round to oval structures result from degeneration of smooth muscle actin fibers, and transition forms can be seen. Stromal hyaline bodies stain with Masson trichrome and PAS, but they do not stain with phosphotungstic acid–hematoxylin (PTAH), methyl green pyronine, Feulgen, Alcian blue at pH 2.5, or Congo red stain. Similar inclusions are characteristic of infantile digital fibromatosis and may be seen in phyllodes tumor of the breast.103 The seminal vesicles arise during the thirteenth week of development as outpouchings of the lower mesonephric ducts. They are bounded by the prostate distally, the base of the bladder anteriorly, and Denonvilliers fascia and the rectum posteriorly. Their anatomic distribution in this region is variable, and they are occasionally found within the capsule of the prostate gland. The seminal vesicles may be palpable on digital rectal examination, and, when intimately associated with the prostate, may be mistaken for prostatic nodularity or induration. Up to 20% of prostate biopsy specimens for nodularity contain fragments of seminal vesicle epithelium, a potential source of diagnostic confusion.70 The mucosa displays complex papillary folds and irregular convoluted lumina, and the lining consists of a nonciliated pseudostratified tall columnar epithelium. The cells are predominantly secretory, with microvesicular lipid droplets and characteristic lipofuscin pigment granules.104 The pigment is golden brown and refractile, increasing in amount with age. The muscular wall consists of a thick circumferential coat of smooth muscle and is thought to serve a contractile function. The ducts of the seminal vesicles merge with the vas deferens on each side to form the ejaculatory ducts and then enter the central zone of the prostate and converge prior to termination at the verumontanum and prostatic urethra (Figs. 8-16 and 8-17). Abnormalities of the seminal vesicles and ejaculatory ducts are covered elsewhere. Fig. 8-16 Ejaculatory duct near origin in the seminal vesicles. Note focal smooth muscle hyperplasia distorting lumen. Cowper glands are small, paired bulbomembranous urethral glands that may be mistaken for prostatic carcinoma in biopsy specimens.105,106 These glands are composed of lobules of closely packed uniform acini lined by cytologically benign cells with abundant apical mucinous cytoplasm (Fig. 8-18). Nuclei are small, solitary, punctate, and basally located, and nucleoli are inconspicuous. Cowper glands are embedded in smooth muscle, thus mimicking the infiltrative pattern of prostatic cancer. Misdiagnosis can be avoided if samples display immunoreactivity for mucin and smooth muscle actin and are negative for PSA and PAP. Fig. 8-18 Cowper gland. A, This lobulated small gland contains multiple small acini with abundant mucinous cytoplasm and small hyperchromatic basal nuclei. B, These acini surround a central duct. Disorders of Cowper glands are exceedingly rare. Urethral syringocele is cystic dilatation that may cause voiding dysfunction in children; it is not apparently associated with other congenital anomalies.107 Carcinoma of Cowper glands is also exceedingly rare and is characterized by frank anaplasia of tumor cells. The most important immunohistochemical markers in prostate pathology are PSA, PAP, high-molecular-weight keratin 34βE12, p63, racemase (P504S), c-myc, and ERG. Androgen receptor immunostaining has not achieved routine clinical utility because of variable results in multiple studies. Standardization of methods of staining and quantitation is recommended to avoid variable results.108 These markers are briefly presented here, but they are discussed at length elsewhere. Immunohistochemical expression of PSA is useful for distinguishing high-grade prostate cancer and urothelial carcinoma, colonic carcinoma, granulomatous prostatitis, and lymphoma.60 PSA also facilitates identification of site of tumor origin in metastatic adenocarcinoma. A list of extraprostatic tissues and tumors that reportedly express PSA immunoreactivity is shown in Table 8-4.109–112 Table 8-4 Immunoreactivity of prostate-specific antigen in extraprostatic tissues and tumors* *Caveat: In many of these tissues and tumors, staining may be patchy, weak, or equivocal. Many of these reports have not been confirmed or validated. Also, contemporary antibodies to prostate-specific antigen may have different specificity and sensitivity from those used in some of these studies. PSA can be detected in frozen sections, paraffin-embedded sections, cell smears, and cytologic preparations of normal and neoplastic prostatic epithelium (Fig. 8-19). In the normal and hyperplastic prostate, PSA was uniformly present at the apical portion of the glandular epithelium of secretory cells. The intensity of the staining decreased in poorly differentiated adenocarcinoma.110,113 Staining is invariably heterogeneous. Microwave antigen retrieval is usually not necessary, even in tissues that have been immersed in formalin for years. Formalin fixation is optimal for localization of PSA, and variation in staining intensity is only partially the result of fixation and embedding effects.114 Immunoreactivity is preserved in decalcified specimens and may be enhanced. PAP is a valuable immunohistochemical marker for identifying prostate cancer when it is used in combination with stains for PSA.115 In the normal and hyperplastic prostate, PAP was uniformly present at the apical portion of the glandular epithelium of secretory cells. There was more intense and uniform staining of cancer cells and the glandular epithelium of well-differentiated adenocarcinoma, whereas less intense and more variable staining was seen in moderately and poorly differentiated adenocarcinoma. The intensity of PAP immunoreactivity correlated with patient survival, probably because of greater androgen responsiveness in immunoreactive cancers.116,117 A list of extraprostatic tissues and tumors that reportedly express PAP immunoreactivity is shown in Table 8-5.109,110,118,119 Table 8-5 Immunoreactivity of prostatic acid phosphatase in extraprostatic tissues and tumors* *Caveat: in many of these tissues and tumors, staining may be patchy, weak, or equivocal. Many of these reports have not been confirmed or validated. Also, contemporary antibodies to prostatic acid phosphatase may have different specificity and sensitivity from those used in some of these studies. Basal cell–specific antikeratin 34βE12 stains virtually all the normal basal cells of the prostate; no staining occurs in the secretory and stromal cells. Basal cell layer disruption is present in 56% of cases of high-grade PIN, more commonly in glands adjacent to invasive carcinoma than in distant glands. The amount of disruption increases with increasing grades of PIN, with loss of more than one third of the basal cell layer in 52% of foci of high-grade PIN. Early carcinoma occurs at sites of acinar outpouching and basal cell layer disruption.34 Prostate cancer cells do not react with this antibody, although it may stain other cancers. Basal cell layer disruption also occurs in inflamed acini, atypical adenomatous hyperplasia, and postatrophic hyperplasia.34,120–123 Despite the clinical utility of high-molecular-weight keratin, caution is urged in interpretation because of the need to rely on negative results to separate adenocarcinoma from its mimics. Numerous confounding factors can interfere with staining, including poor tissue preservation and fixation and lack of enzyme predigestion.124 p63 is a nuclear protein that is among the best of the diagnostically useful basal cell markers. p63 staining of nuclei was reported to be at least as sensitive and specific for the identification of basal cells in diagnostic prostate specimens as high-molecular-weight cytokeratin staining of cytoplasm (Fig. 8-20).125 p63 may be more sensitive than 34βE12 in staining benign basal cells, particularly in transurethral resection of the prostate (TURP) specimens, and it may offer advantage over 34βE12 in diagnostically challenging cases.123,126 The basal cell cocktail (34βE12 and p63) increased the sensitivity of basal cell detection and reduced staining variability.127,128 The p63 gene is also expressed in respiratory epithelia, breast and bronchial myoepithelial cells, cytotrophoblast cells of human placenta, scattered cells of lymph nodes and germinal centers, and squamous cell carcinoma of the lung.26,129 Triple staining with racemase, high-molecular-weight cytokeratin, and p63 is used by many laboratories for the diagnosis of prostate cancer.26,123,130 The addition of c-myc to this trio is routine in our laboratory (discussed later). α-Methylacyl–coenzyme A (CoA) racemase (AMACR, racemase) gene product, also referred to as P504S protein, is an enzyme involved in β-oxidation of branched-chain fatty acids. It is a novel tumor marker for several human cancers and their precursor lesions, including prostate cancer.128,131–133 Racemase results are positive in approximately 80% of small prostate cancers on needle biopsy and are less intense and more heterogeneous in unusual morphologic variants of prostate cancer, including atrophic, foamy gland, and pseudohyperplastic cancers (see Fig 8-20; Table 8-6); in one report, 91% of irradiated prostate cancers retained racemase immunoreactivity.134 Androgen deprivation therapy decreases racemase immunoreactivity in prostate cancer.26,135 Positive racemase staining is also found in the majority of cases of high-grade PIN and in 10% to 15% of cases of atypical adenomatous hyperplasia and occasional benign glands (see Fig. 8-20); it is rare in seminal vesicle epithelium.132,133,136–138 Nephrogenic adenoma is an important mimic of malignancy that is strongly positive for racemase; this must be kept in mind in interpretation of small foci in needle biopsies. Jiang and colleagues found that the sensitivity and specificity of racemase immunodetection of prostatic adenocarcinoma were 97% and 92%, respectively; positive and negative predictive values were 95%. Racemase immunostaining intensity and percentage in prostatic adenocarcinoma were significantly higher than were those in benign prostatic tissue.139 In our experience, racemase results are negative in up to 20% of cancer cases. A compelling need exists for an immunohistochemical stain for cancer nuclei that would provide assistance in cases in which racemase staining is marginal or absent.140 Intense nuclear staining of c-myc was identified in epithelial cells in 15%, 100%, and 97% of cases of benign tissue, PIN,141 and cancer, respectively; the mean percentage of c-myc–positive cells was 0.2% (range, 0% to 5%), 34.4% (range, 10% to 50%), and 32.3% (range, 5% to 70%), respectively. The c-myc gene was highly overexpressed in prostate cancer cells, especially in those with negative racemase staining. Cocktail staining of racemase, high-molecular-weight cytokeratin, p63, and c-myc on a single slide is now our standard for the workup of difficult prostate needle biopsies.26,122,123,142–146 Negative immunohistochemical stain for basal cells is not diagnostic of carcinoma by itself because occasional benign glands may not show immunoreactivity, so positive immunohistochemical markers specific for prostate cancer, such as racemase and c-myc, are of great value in confirming malignancy. Positive racemase and c-myc staining converts an atypical diagnosis, based on suspicious histologic features and negative basal cell marker stains, to cancer in approximately 10% of cases thought to be atypical by contributing pathologists and in approximately 50% of cases thought to be atypical by a specialist in genitourinary pathology. We use the cocktail staining protocol in routine practice and find it to be very useful for the diagnosis of small prostate cancers (see Fig. 8-20). Optimizing the staining conditions for cocktail antibodies is very important for staining interpretation.137,147 Although it has limitations with respect to sensitivity and specificity, racemase has rapidly become a standard adjunctive stain used to reach a definitive diagnosis in prostate biopsy specimens considered to be atypical but not diagnostic of malignancy on H&E-stained sections alone.147–150 TMPRSS2-ERG, the most common gene fusion in prostate cancer, is associated with expression of a truncated protein product of the oncogene ERG. A novel anti-ERG monoclonal antibody has been characterized,151 and immunohistochemical ERG expression was highly concordant with the ERG mRNA overexpression (sensitivity, 100%; specificity, 85%). ERG overexpression was the result of TMPRSS2-ERG gene fusion in all cases. ERG protein expression was identified in 52% of cases of high-grade PIN and in 61% of adenocarcinomas on needle biopsies; conversely, only 6% of benign acini adjacent to cancer displayed weak staining in the secretory cells (Fig. 8-21). Prostate-specific membrane antigen (PSMA) is a membrane-bound antigen that is highly specific for benign and malignant prostatic epithelium, although endothelial cells in multiple organs are also immunoreactive. Cytoplasmic epithelial immunoreactivity for PSMA is intense.152–155 Investigators reported that the number of immunoreactive cells increased from benign epithelium to high-grade PIN and prostatic adenocarcinoma. The most extensive and intense staining for PSMA was observed in high-grade carcinoma, with immunoreactivity in virtually every cell in Gleason primary patterns 4 or 5 (Table 8-7).156 Extraprostatic expression of PSMA is highly restricted other than for vascular staining, and nonprostatic cancer, including renal cell carcinoma, urothelial carcinoma, and colonic adenocarcinoma, is invariably negative for PSMA. PSMA immunoreactivity in cancer cells was not predictive of PSA biochemical failure or recurrence in a cohort of organ-confined margin-negative cancers treated by surgery157; these findings differ from serum studies in which elevated concentrations of PSMA indicated surgical treatment failure.158–160 PSMA is clinically useful for diagnostic and therapeutic applications. PSMA is expressed in lymph node152,153 and bone marrow metastases153 of prostate cancer (Fig. 8-22), thus underscoring its utility in identifying cancer of an unknown primary site. Serum PSMA was of prognostic significance, especially in the presence of metastases, and correlated well with cancer stage in a screened population. Despite its potential utility, PSMA immunohistochemistry is rarely used in routine practice. Table 8-7 Comparative immunoreactivity of prostate-specific membrane antigen and prostate-specific antigen in the benign and neoplastic prostate in 184 radical prostatectomies The human kallikrein family consists of three members: hK1, hK2, and hK3 (PSA). The mRNA for hK2 and PSA is located predominantly in prostatic epithelium and is regulated by androgens.161–163 In addition, hK2 has 78% amino acid homology with PSA and is expressed predominantly in the prostate, a finding suggesting that it may be a clinically useful marker for the diagnosis and monitoring of prostate cancer.162–165 The intensity and extent of hK2 expression were greater in cancer than in PIN and were greater in PIN than in benign epithelium. Gleason primary grade 4 and 5 cancers showed hK2 staining in almost every cell, whereas heterogeneity of staining was greater in lower grades of cancer.166 In marked contrast to hK2, PSA and PAP immunoreactivity was most intense in benign epithelium and stained to a lesser extent in PIN and carcinoma.166,167 The number of immunoreactive cells for hK2 and PSA was not predictive of cancer recurrence.168 Tissue expression of hK2 appears to be regulated independently of PSA and PAP.169 Numerous immunohistochemical markers have been identified in the prostate, and many of these are preferentially found in the basal cell layer of the epithelium (Table 8-8). Basal cells display immunoreactivity at least focally for keratins 5, 10, 11, 13, 14, 16, and 19; of these, only keratin 19 is also found in secretory cells.129 Keratins found exclusively in the secretory cells include 7, 8, and 18. Expression of S100-A6 (calcyclin), a calcium-binding protein, is restricted in the prostate to the basal cells of benign glands but not in cancer.170,171 Table 8-8 Immunophenotype of prostatic basal cells
Nonneoplastic diseases of the prostate
Embryology and fetal-prepubertal history
Anatomy and histology of the prostate
Capsule
Prostatic urethra and verumontanum
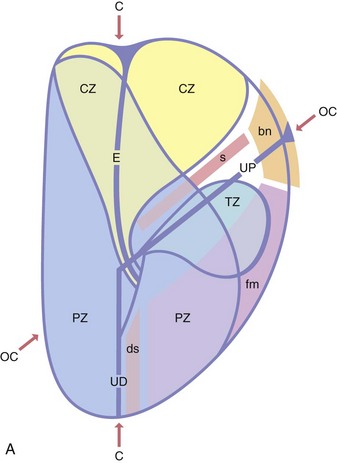
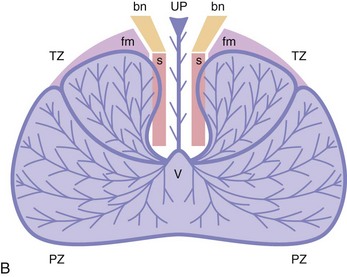
Zonal anatomy of the prostate
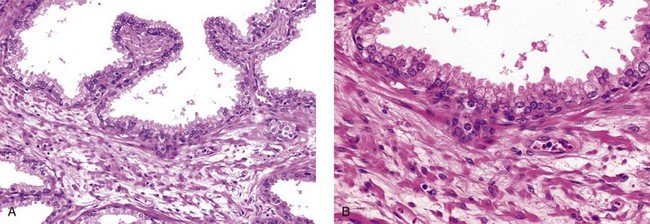
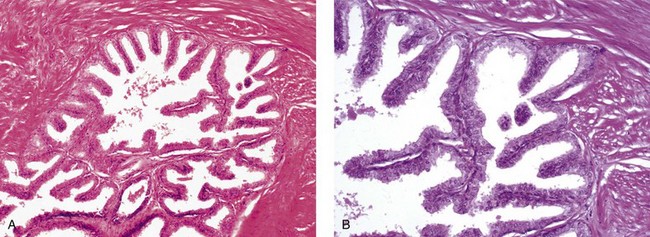
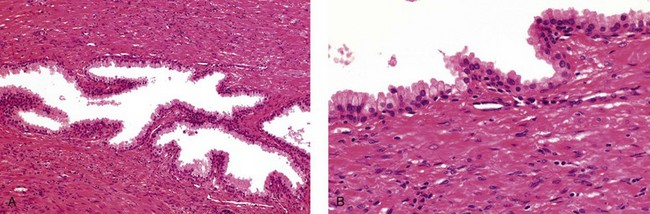
Nerve supply
Blood supply
Normal epithelium of the prostate
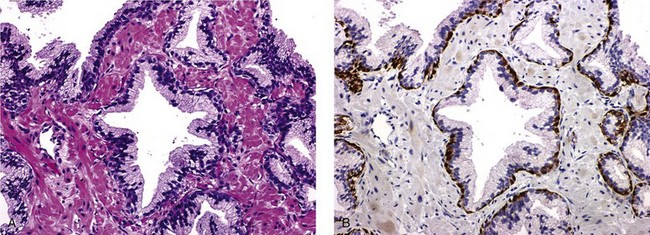
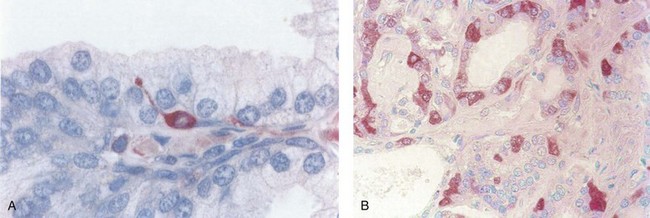
Pigment
Luminal products
Stromal hyaline bodies
Seminal vesicles and ejaculatory ducts
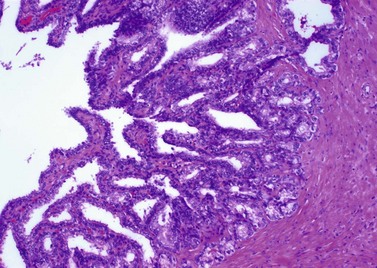
Cowper glands
Immunohistochemistry
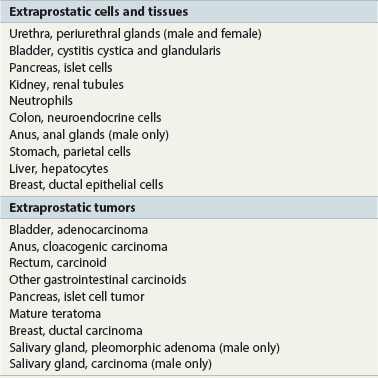
Prostate-specific antigen
Prostatic acid phosphatase
Keratin 34βE12 (keratin 903; high-molecular-weight keratin)
p63
α-Methylacyl–coenzyme A racemase/P504S (AMACR, P504S, racemase)
c-myc
Combination of racemase, keratin 34βE12, p63, and c-myc
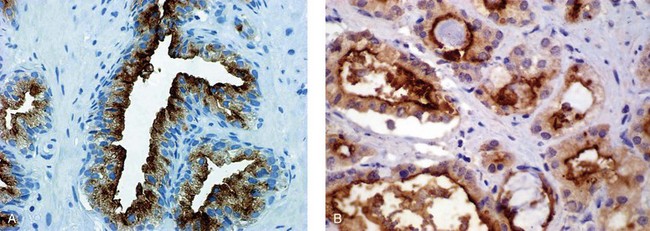
ERG
Prostate-specific membrane antigen
Percentage (%) of immunoreactive cells + SD (range)
PSMA
Benign
69.5 + 17.3 (20-90)
High-grade PIN
77.9 + 13.7 (30-100)
Cancer
80.2 + 13.7 (30-100)
PSA
Benign
81.3 + 11.8 (20-90)
High-grade PIN
64.8 + 17.3 (10-90)
Cancer
74.2 + 16.2 (10-90)
Human glandular kallikrein 2
Other markers of basal cells
Biomarker
Function
Findings
Proliferating cell nuclear antigen
Cell proliferation marker
Up to 79% of labeled cells are basal cells
MIB1
Cell proliferation marker
Up to 77% of labeled cells are basal cells
Ki67
Cell proliferation marker
Up to 81% of labeled cells are basal cells
Androgen receptors
Nuclear receptors that are necessary for prostatic epithelial growth
Strong immunoreactivity; also present in cancer cells ![]()
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree

 Get Clinical Tree app for offline access
Get Clinical Tree app for offline access