Symptoms
Signs
Laboratory features
Common
– None
– Hepatomegaly
– Two- to fourfold ↑ of serum ALT and AST
– AST/ALT ratio <1 usually
– Alkaline phosphatase level ↑
– Normal bilirubin, albumin levels, and prothrombin time
– ↑ Serum ferritin level
Uncommon
– Vague RUQ pain
– Fatigue
– Malaise
– Splenomegaly
– Spider angiomata
– Palmar erythema
– Ascites
– Low-titer (less than 1:320) ANA
As clinical, laboratory, and liver biopsy findings are similar in alcoholic liver disease, the diagnosis of NAF LD can only be made in the absence of significant alcohol use. This is typically defined as the consumption of less than 20–40 g of alcohol per day. Other conditions that lead to hepatic steatosis independent of NAFLD are seen less commonly than alcoholic liver disease, but still should be considered. Hepatic steatosis can occur in conditions resulting in rapid weight loss as seen with total parenteral nutrition, extensive small bowel resection, biliopancreatic diversion, or jejunoileal bypass. Medications such as amiodarone, valproic acid, methotrexate, tamoxifen, glucocorticoids, certain antiretrovirals, and tetracyclines as well as systemic conditions such as Wilson disease, abetalipoproteinemia, and lipodystrophy can also produce hepatic steatosis.
A mild-to-moderate (1.5–4-fold) elevation of the serum aspartate aminotransferase (AST) or alanine aminotransferase (ALT) level, or both, is common, although levels exceeding ten times the upper limit of normal are rare. A mean ALT of 83 and AST of 63 IU/mL was shown in a large retrospective study of NAFLD patients [7]. It is important to recognize that NAFLD can occur with completely “normal” LAEs and laboratory findings do not always correlate with the histologic severity of NAFLD. The entire histologic spectrum of NAFLD, including cirrhosis, can be seen in patients with normal or near-normal serum aminotransferase levels [8]. In most NAFLD patients, the serum ALT level usually is greater than the AST level, particularly in comparison to alcoholic liver disease where AST is typically twofold higher than ALT. The alkaline phosphatase and gamma-glutamyl transpeptidase (GGT) levels may also be elevated, and occasionally, NAFLD can present as an isolated alkaline phosphatase elevation, more often in female patients [9]. The serum bilirubin level, prothrombin time, and serum albumin level are usually normal, except in patients with NAFLD-associated cirrhosis.
Antinuclear antibodies (ANA) can be elevated in up to one fourth of patients with NAFLD, although typically in low titers less than 1:320. Laboratory tests for other chronic liver disease are negative [10], although NAFLD can coexist in patients with hepatitis C infection (HCV). In HCV and in one study of 628 adult NAFLD patients, serum ferritin > 1.5 × upper limit of normal was independently associated with a higher NAFLD activity score (NAS) [11]. Iron overload in NAFLD populations appears to be secondary and limited to Kupffer cells, with a subsequent study demonstrating that the frequency of genetic hemochromatosis was not increased in NAFLD patients [12]. This is consistent with the belief that increased serum iron indices are a by-product of hepatic inflammation, rather than a direct contributor to the pathogenesis of NAFLD. A recent study supported this argument and demonstrated that 6 months of phlebotomy with the goal of lowering serum ferritin did not change serum markers of inflammation or LAEs in a study of 74 NAFLD patients [13]. Alternatively, two older small studies have suggested that iron depletion may have a therapeutic role in NAFLD by decreasing plasma insulin, glucose, and serum aminotransferase levels [14, 15]. Further study is likely necessary to clarify if there is any therapeutic benefit to phlebotomy, but there is consensus that elevated ferritin is associated with increased disease activity (although not necessarily fibrosis) in NAFLD populations.
In the absence of a liver biopsy, it is difficult to distinguish those patients with NASH as the most clinically relevant subset of patients with NAFLD in terms of liver-specific outcomes. As previously mentioned, the clinical picture and symptom profile are decidedly unhelpful in elucidating which patients meet criteria for NASH. Noninvasive tests to include laboratory and radiographic modalities as well as scoring systems will be discussed elsewhere, although they generally have proven most useful in identifying advanced fibrosis. A clinical presentation of NAFLD that includes borderline low platelets or an AST:ALT ratio approaching 1 increases the clinical likelihood of advanced fibrosis and should warrant further work-up either with noninvasive testing or a liver biopsy.
Clinical Associations
NAFLD is associated with a wide variety of clinical conditions, and often their coexistence has clinical implications (Fig. 9.1). The most studied and well-founded association is that of NAFLD with diabetes mellitus (DM). NAFLD and specifically nonalcoholic steatohepatitis (NASH) are often associated with DM where a 60–76 % prevalence of NAFLD and a 22 % prevalence of NASH have been reported [16]. NAFLD appears to increase the risk of developing DM [17], and DM is an independent risk factor for NAFLD [18]. The presence of both conditions has significant clinical implications as it is predictive of increased risk of death from both liver-related and all-cause mortalities [19]. NAFLD also has been associated with higher rates of end-stage organ damage from DM as evidenced by proliferative retinopathy and increased rates of chronic kidney disease (CKD) [20].
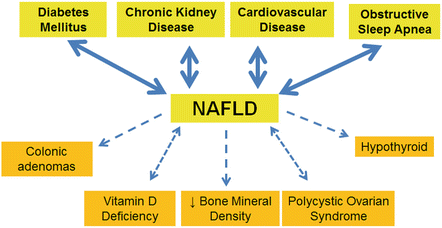

Fig. 9.1
Conditions associated with NAFLD. This figure illustrates conditions associated with NAFLD. A bidirectional arrow indicates a mutual association. A dashed arrow indicates mixed data but at least some association with direction of arrow indicating prevailing relationship
CVD comes in a strong second with numerous studies showing increased rates of CVD and cardiovascular events in NAFLD patients. Carotid artery intima-media thickness has been shown to be increased in NAFLD patients [21], and a meta-analysis of eight studies confirmed NAFLD as an independent risk factor for CVD with an OR of 2.05 (95 % CI, 1.81–2.31) [22]. CVD is the primary cause of death in NAFLD patients, but the mechanisms defining this relationship are still under investigation. Epicardial fat has been recently shown to be increased in NAFLD patients and proportionally related to hepatic fibrosis so this may partially explain the association [23]. Other potential explanations for the link between NAFLD and CVD include inflammation, oxidative stress, insulin resistance, dyslipidemia, endothelial dysfunction, and cytokine imbalances [24]. Interestingly, the risk for CV events persists even after liver transplant with the largest study to date demonstrating a 4.12 OR (95 % CI, 1.91–8.90) for CV events in NAFLD patients compared to patients transplanted for alcoholic liver disease [25].
Obstructive sleep apnea (OSA) has also been shown to be prevalent in NAFLD populations at rates higher than the estimated general OSA prevalence of 1–4 % or even the 25–35 % shown in obese populations [26, 27]. Fifty percent of NAFLD patients have symptoms suggestive of OSA, and 90 % of obese patients with OSA have NAFLD [28]. There is even some evidence to suggest an association of the chronic intermittent hypoxia seen in OSA with NAFLD disease severity. LAE elevation in a cohort of patients with steatosis on ultrasound was shown to be related to the oxygen desaturation index that is used to determine severity of OSA [29]. A study of morbidly obese patients undergoing bariatric surgery demonstrated OSA was associated with a high NAS and increased fibrosis [30]. This was also shown in a study of pediatric patients which demonstrated increased fibrosis in NAFLD patients with OSA [31]. It is less clear whether treatment of OSA with continuous positive airway pressure (CPAP) or other modality would benefit NAFLD. One study of obese males did demonstrate an improvement in LAEs with CPAP [32], although this was not confirmed by a similar subsequent study [33]. Histologic outcomes were absent from both of these studies, and larger randomized controlled trials with histologic end points are required to definitely evaluate the effectiveness of OSA treatment in NAFLD.
CKD has long been associated with hypertension and DM and is common in the general population with reported prevalence rates ranging from 4.3 to 13 % [34, 35]. Higher prevalence rates on the order of 21–54 % have been demonstrated in NAFLD populations where CKD was defined by a decrease glomerular filtration rate (GFR) ≤60 mL/min/1.73 m2, overt proteinuria, or microalbuminuria with a urinary albumin/creatinine ≥30 mg/g [36]. In seven studies, NAFLD was independently associated with CKD after adjusting for age, sex, body mass index, hypertension, DM, smoking, and hyperlipidemia. One important caveat was that most of these did not use liver biopsy to evaluate for NAFLD and instead relied on LAEs or ultrasound (US). Despite the preponderance of evidence linking CKD and NAFLD, one large cross-sectional study using NHANES data from 1988 to 1994 did not show an association of CKD with a US diagnosis of NAFLD after adjusting for components of the metabolic syndrome [37]. A subsequent NHANES analysis from 2001 to 2006 showed mild elevation of GGT was associated with an increased prevalence of CKD [38] and was further supported by three hospital-based studies using liver biopsy to evaluate for NAFLD [39–41]. These smaller studies were strengthened by their histologic data and generally showed NAFLD, and in some instances NASH or advanced fibrosis, to be associated with CKD. The association of NAFLD and CKD was confirmed by the large meta-analysis conducted by Musso et al. that contained 33 studies with over 63,000 participants, and the severity of each diagnosis was increased in the presence of the associated diagnosis [42].
The evidence demonstrating that a diagnosis of NAFLD carries an increased risk of incident CKD is equally compelling. Four of the five studies to date demonstrated that NAFLD was independently associated with the development of de novo CKD, although it is notable that three of the five studies used elevated GGT to diagnose NAFLD [43–47]. The four positive studies demonstrated HRs ranging from 1.49 to 4.38 for the risk of developing CKD in the presence of NAFLD.
The relationship of CKD and NAFLD in the setting of cirrhosis necessitating liver transplant requires special mention as the number of transplants for NASH cirrhosis increases. A myriad of issues this presents are addressed in a recent editorial by Musso et al. which summarized the rising rates of liver transplant for NASH cirrhosis along with increasing postoperative issues with CKD and renal failure [48]. The need for dual organ transplantation (liver and kidney) was also notable in this population, and chronic kidney disease has been shown to be associated with increased mortality in liver transplant [49].
In summary, NAFLD and CKD are strongly associated with abundant evidence linking these two diagnoses and their respective disease severities. Patients diagnosed with NAFLD should be evaluated for CKD, and conversely those with CKD, evaluated for NAFLD.
The relationship between vitamin D and NAFLD has also been the focus of extensive investigation with most evidence suggesting vitamin D deficiency (VDD) was found more commonly in NAFLD populations [50]. VDD has shown increased and widespread prevalence in recent years similar to that of NAFLD prevalence rates, although the coexistence of NAFLD and VDD appears to go beyond a simple association. VDD is found in populations with NAFLD at higher rates than matched controls when using data from NHANES II [51]. Further study has shown this association to be independent of age, gender, and triglyceride or glucose levels [52]. The association of VDD and disease severity is more controversial, although one study showed lower VDD levels in NASH patients compared to isolated fatty liver [53]. This was not substantiated by a subsequent study [54] and definitive evidence is still required. There are no prospective studies to suggest that vitamin D replacement may improve NAFLD or NASH, although it is reasonable to check vitamin D levels and replete as necessary in known NAFLD patients.
Bone mineral density (BMD) in the setting of NAFLD has also been investigated with most studies suggesting an inverse relationship. A study of postmenopausal Korean women demonstrated lower BMD in US-defined NAFLD even with adjustment for BMI, smoking, age, alcohol use, and the metabolic syndrome [55]. This was also shown in a male Chinese population where those with US-diagnosed NAFLD were 2.5 times more likely to have an osteoporotic fracture [56] and in a smaller study of children, where 45 % with biopsy-proven NAFLD had low BMD compared to 0 % of age- and weight-matched controls [57]. No data exists as to whether or not osteoporosis is associated with advanced histology in NAFLD. Similar to VDD, a diagnosis of NAFLD should increase suspicion for coexistent osteoporosis, although it is too early to advocate universal BMD testing in this large population.
Components of the MS including obesity, insulin resistance, and dyslipidemia have also been shown to be associated with increased prevalence of colonic adenomas. It is therefore not surprising that several retrospective studies and one prospective study have demonstrated a relationship between NAFLD and colonic adenomas. The two largest studies to date, both in Asian populations, showed a higher prevalence of colonic adenomas as well as advanced neoplasia such as cancer, high-grade dysplasia, or villous histology in NAFLD populations [58, 59]. In these two studies, NASH histology was more strongly correlated to adenoma detection than non-NASH NAFLD. The largest American study to date confirmed an association of adenomatous polyps in NAFLD compared to non-NAFLD populations, but this did not correlate to histology [60]. The association of adenomatous polyps and NAFLD did not appear to extend to colorectal cancer (CRC) where 227 patients with CRC were followed (27 % with NAFLD) and outcomes were similar among NAFLD and non-NAFLD groups [61].
Polycystic ovarian syndrome (PCOS) is another condition that has been associated with NAFLD. Markedly increased rates of NAFLD in PCOS patients with OSA (83.3 % vs. 26.9 %, p < 0.01) have been demonstrated [62] as well as in upward of 15 % of obese adolescent females [63]. Subsequent study associated the increased risk of PCOS patients for NAFLD in a manner independent of BMI [64]. A cross-sectional Australian study has provided evidence that the converse is also true: NAFLD patients are at increased risk of PCOS. In this small study, ten of 14 patients with US- or biopsy-proven NAFLD had PCOS which translated to a 71 % prevalence, significantly higher than a similar female population [65]. At a minimum, NAFLD should be considered in all PCOS patients, particularly those with elevated LAEs, and female NAFLD patients with gynecologic symptoms should be evaluated for PCOS.
Other endocrine-related disorders associated with NAFLD include hypothyroidism, growth hormone deficiency, hypogonadism, hypopituitarism, and hypercortisolemia. The data is most abundant linking NAFLD with higher prevalence of hypothyroidism. Biopsy-proven NAFLD has been associated with a 21 % prevalence of hypothyroidism compared to 9.5 % of age-, sex-, ethnicity-, and BMI-matched controls [66]. A larger study also confirmed this association in patients with both overt and subclinical hypothyroidism in a manner independent of known metabolic risk factors [67].
Another association with NAFLD that has been seen at least in preliminary studies is elevated uric acid levels. The association of elevated uric acid and NAFLD was demonstrated in a cohort of 528 Chinese postmenopausal women of normal BMI [68] which was confirmed in group of biopsy-proven male NAFLD patients [69]. Further study is required to determine if elevated uric acid levels translate to clinically significant gout and whether hyperuricemia is associated with increased disease severity in NAFLD.
In total, NAFLD is associated with a myriad of extrahepatic conditions, most of which are also related to the MS. The association of NAFLD with intra- and extrahepatic malignancy is discussed elsewhere in this text but is also thought to be related to inherent risk from some component of the MS or obesity.
Natural History
The natural history of NAFLD is highly variable, particularly since disease progression does not always follow a linear course. Histopathology remains critically important, and even though the accepted paradigm of non-NASH NAFLD versus NASH is overly simplistic, this distinction is still an important predictor of natural history and outcomes. Obtaining a liver biopsy allows for the identification of features such as lobular and portal inflammation and hepatocyte ballooning that enables a pathologist to distinguish non-NASH NAFLD from NASH, and in addition, it allows for the quantification of fibrosis. The prognosis in patients with steatosis, none to mild nonspecific hepatocellular inflammation, and no fibrosis (non-NASH NAFLD) has been thought to be favorable with minimal potential for histologic or clinical progression [70, 71]. Most non-NASH NAFLD patients are thought to have similar mortality rates to the general population, while an established diagnosis of NASH predicts a reduced life expectancy from cardiovascular, malignancy, or liver-related causes [72, 73]. Fortunately, it is estimated that approximately 70–75 of adult NAFLD patients will fit into the non-NASH NAFLD category [74] (Fig. 9.2).
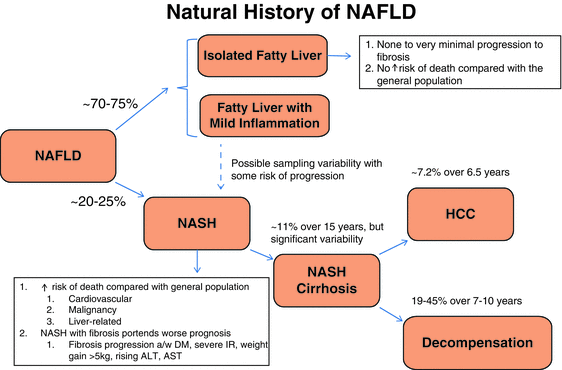

Fig. 9.2
Natural history of NAFLD. The progression of cirrhosis to end-stage liver disease is 39–62 % in patients with NASH. Of these patients, 22–33 % will experience liver disease-related mortality. The survival time of these patients is 5–7 years. The development of ascites is the most common liver-related morbidity. The mortality rate is higher than in the general population (standard mortality ratio, 1.34; 95 % CI: 1.003–1.76; p = 0.03) (Adams LA et al. Gastroenterol. 2005; 129:113–121 abst). The study by Sanyal et al. compared outcomes in patients with cirrhosis in NASH vs. cirrhosis in HCV, using the Child-Turcotte-Pugh (CTP) score. The CTP score assesses the severity of liver disease by scoring bilirubin, ascites, prothrombin time (INR), encephalopathy grade, and serum albumin. NASH patients with CTP class A had a lower mortality rate than HCV patients. Mortality rates were similar in NASH and HCV at CTP class B and higher. These results corroborate the Hui et al. study which found that NASH-associated cirrhosis had a similar prognosis to HCV cirrhosis. Modified from Torres DM et al. [2], with permission of Elsevier
Recent evidence from a natural history study following 108 NAFLD patients for a median of 6.6 years revealed 44 % of non-NASH NAFLD patients progressed to NASH and 22 % alarmingly progressed to stage 3 fibrosis [75]. Overall, 37 % of non-NASH NAFLD and 43 % of NASH patients from this cohort had some degree of fibrosis progression during follow-up. While this relatively small study does not provide definitive evidence, it certainly calls into question the previous dogma that non-NASH NAFLD does not progress to NASH or lead to significant fibrosis. Further evidence that non-NASH NAFLD can lead to hepatic fibrosis was also revealed in a meta-analysis of 11 cohort studies that included 411 patients with biopsy-proven NAFLD. Non-NASH NAFLD patients with stage 0 fibrosis at baseline progressed 0.07 stages versus 0.14 stages annually in NASH patients [76]. The authors translated this into one stage of fibrosis progression over 14.3 years for a non-NASH NAFLD patient and 7.1 years for a patient with NASH. Another key finding was the significant variability in fibrosis progression rates with approximately one in five patients demonstrating rapid fibrosis progression, although due to limited data, the unifying factors associated with rapid progression could not be identified. These findings are somewhat consistent with the previous teaching that one third of NASH patients improve, one third stay the same, and one third worsen with the additional caveat that non-NASH NAFLD patients may also be susceptible to disease progression.
There is growing evidence that the non-NASH NAFLD paradigm is too broad in scope as it relates to prognosis and natural history. Data suggest that non-NASH NAFLD can possibly be further divided into an isolated steatosis group, having no inflammation, and a group defined by fatty liver and mild inflammation, or “indeterminate NASH” as proposed in a recent editorial [77]. Despite the mild inflammation, ballooning is not apparent and thus a diagnosis of NASH cannot be made. This further delineation of NAFLD patients is supported by a recent study showing that isolated steatosis patients progress much more slowly than patients with steatosis and mild inflammation [78] and two prior studies that demonstrated that isolated steatosis patients did not progress at all [72, 79].
With preeminence of NASH versus non-NASH NAFLD now in question based on recent data, the importance of fibrosis in predicting outcomes has remained. NASH with fibrosis suggests a worse prognosis than NASH without fibrosis [80]. Some studies have suggested fibrosis is the most important predictor of outcome exceeding the NAS which includes necroinflammation, ballooning, and degree of steatosis. A large natural history study of 229 biopsy-proven NAFLD patients followed for a mean of 26.4 years (±5.6, range 6–33) demonstrated that NAFLD with fibrosis portended a worse prognosis, while the NAS was not helpful in natural history determination [81]. Increased fibrosis stages 3–4, regardless of the NAS, had substantially increased mortality with a HR 3.3 (CI 2.27–4.76, p < 0.001). Conversely, a high NAS in the absence of advanced fibrosis did not predict outcomes. While this study did not distinguish NASH from non-NASH NAFLD, the authors grouped patients into NAS 0–4 or 5–8 as a (suboptimal) surrogate. A NAS of 0–4 or 5–8 in the absence of advanced fibrosis (stage 0–2) did not predict increased overall mortality with an HR of 1.41 (CI 0.97–2.06, p = 0.07) and HR 1.13, respectively (95 % CI 0.79–1.60, p = 0.51). The caveat to this was two patients with a NAS 0–4 died due to cirrhosis-related complications, although both patients had stage 2 fibrosis. The authors explained these findings in the discussion suggesting that the NAS was overly reliant on hepatic steatosis to which it gives equal importance alongside ballooning and lobular inflammation.
Additional factors associated with fibrosis progression include the presence of DM, severe insulin resistance, cigarette smoking, weight gain greater than 5 kg, or rising ALT and AST levels [82, 83]. As previously mentioned, fibrosis progression rates are variable, and no clinical or laboratory data has been shown to reliably predict disease course. One author suggested that ~11 % of NASH patients progress to cirrhosis over a 15-year period [84]. Interestingly, despite the similarities in NAFLD histology to alcoholic hepatitis, outcomes in NAFLD are much better. The 5-year survival rate of patients with alcoholic hepatitis is only 50–75 %, in large part due to the development of cirrhosis in greater than 50 % with its inherent complications [85].
Cirrhosis secondary to NAFLD has comparable outcomes to other causes of cirrhosis, and most cryptogenic cirrhosis is thought to be NAFLD [86]. NAFLD cirrhosis can lead to hepatocellular carcinoma (HCC) and NAFLD-related HCC is the fast-growing indication for liver transplantation [87, 88]. Five- to ten-year outcomes of NAFLD-associated cirrhosis appear similar to that for HCV-associated cirrhosis [89].
The third most common indication for orthotopic liver transplant (OLT) behind chronic hepatitis C and alcoholic liver disease in America is NASH cirrhosis, although it is expected to become the number one indication for liver transplant with the next 1–2 decades [90]. Long-term survival from a transplant due to NASH cirrhosis is similar to other indications although eligibility for transplant may be limited secondary to coexisting conditions such as heart disease and 30-day transplant mortality is still higher for NASH cirrhosis [91, 92]. The majority of patients have recurrent steatosis 5 years out from transplant, although only 5 % developed recurrent cirrhosis within that time [93]. Recent data distinguished two kinds of posttransplant NAFLD—that of de novo fatty liver disease with an alternative indication for the primary liver transplant and recurrent NAFLD [94]. Recurrent NAFLD in this study had a more aggressive course with 71 % of patients showing stage 3–4 fibrosis after 5 years compared to 12.5 % (p < 0.02) of those with de novo NAFLD. This combined with the previously mentioned almost fourfold increased risk for cardiovascular events posttransplant (compared to transplant for alcoholic liver disease) necessitates the need to develop effective screening methods for NASH cirrhotics to identify those at risk for recurrent NASH or CVD posttransplant.
Conclusion
NAFLD can have a varied clinical presentation with most patients demonstrating an asymptomatic elevation of serum aminotransferases with hepatic steatosis on imaging. Occasionally, symptoms may be what bring that patient to medical attention although it is important to recognize that symptoms do correlate to disease severity or disease progression. Clinical suspicion for NAFLD and in particularly NASH should be heightened in a number of medical conditions associated with the MS. First and foremost, diabetic patients, particularly those with elevated serum aminotransferases, should be evaluated for NAFLD. Obesity, CVD, and OSA are also associated with NAFLD and are thought to increase the likelihood of NASH.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree







