In the evaluation of biliary diseases, cholangioscopy is considered as complementary procedure to radiographic imaging. Direct visualization of the bile duct is the premier advantage of cholangioscopy over indirect imaging techniques. However, cholangioscopy has not gained wide acceptance because of several technical limitations such as scope fragility, impaired steerability, limited irrigation, and suction capabilities, as well as the need for two experienced endoscopists. Recent innovations such as the implementation of electronic video cholangioscopes and the development of single-operator systems facilitate the procedure, and promise to increase the diagnostic and therapeutic yield of cholangioscopy.
In the evaluation of biliary diseases, cholangioscopy is considered as complementary procedure to radiographic imaging by computed tomography (CT) scan, magnetic resonance imaging (MRI), magnetic resonance cholangiopancreatography (MRCP), endoscopic retrograde cholangiopancreatography (ERCP), endoscopic ultrasonography (EUS), and intraductal ultrasonography (IDUS). Direct visualization of the bile duct is the premier advantage of cholangioscopy over these indirect imaging techniques. Peroral cholangioscopy (POCS) and percutaneous transhepatic cholangioscopy (PTCS) improve accuracy in differentiation between benign and malignant processes, allowing targeted sampling of tissue and precise mapping of tumors in preparation for surgical resection. Moreover, cholangioscopy provides endoscopic guidance for therapeutic interventions, such as electrohydraulic lithotripsy (EHL), laser lithotripsy (LL), photodynamic therapy (PDT), and argon plasma coagulation (APC). However, cholangioscopy has not gained wide acceptance because of several technical limitations such as scope fragility, impaired steerability, limited irrigation, and suction capabilities, as well as the need for two experienced endoscopists. Several recent innovations such as the implementation of electronic video cholangioscopes and the development of single-operator systems, including the semidisposable SpyGlass Direct Visualization System and the direct biliary approach with ultraslim upper endoscopes, facilitate the procedure and promise to increase the diagnostic and therapeutic yield of cholangioscopy.
Equipment and techniques
POCS was initially described in 1976. PTCS was also developed in the 1970s. The adoption of these procedures has been slowed in part by technological limitations of the cholangioscopes. One of the first reports demonstrated a fiberscope of 8.8 mm diameter, which was inserted perorally into the biliary system after endoscopic sphincterotomy without the need of a second guiding scope. In the following years the idea of guiding a small-caliber “baby” cholangioscope through the channel of a dedicated larger channel “mother” duodenoscope into the common bile duct (CBD) gained wide acceptance. This “mother-baby” system is operated by 2 experienced endoscopists. The conventional fiberoptic scopes used in the mother-baby system have a distal diameter of 4.5 mm. The scopes have one instrumental channel and the tip deflection is limited to one plane (up-down) of approximately 90° without lateral deflection. The baby scopes are fragile and their optic fibers are prone to break easily from pressure applied with the elevator of the duodenoscope. These scopes require a dedicated light source, image processor, and water-air pump. Finally the baby scope image is projected onto a separate video monitor. Two endoscopists are required and a prior sphincterotomy or balloon sphincteroplasty is usually necessary to insert the baby scope into the CBD. To facilitate ductal intubation, administration of agents that relax the sphincter of Oddi, such as hyoscines, glucagon, and isosorbide dinitrate, have been reported to be helpful. Inserting the baby scope over a guidewire into the duct reduces the need for elevator use and risk of scope damage. Once the scope is advanced to the target location, the guidewire should be removed to permit use of the accessory channel for irrigation and introduction of devices. Several miniscopes have been developed with reduced diameters ranging from 2 to 3.5 mm, allowing insertion thorough conventional therapeutic duodenoscopes and their delivery into even small bile ducts. If the outer diameter is less than 2.5 mm, access without prior sphincterotomy is possible. A fine-caliber flexible miniscope created by Soda and colleagues allowed access to the CBD without sphincterotomy, due to its external diameter of 2.09 mm including, unlike many other miniscopes, a central working channel of 0.72 mm. Similarly, Sander and Poesl have developed a less fragile, steerable new miniscope for peroral cholangioscopy with 2 different degrees of stiffness and 2 channels: a 0.4-mm (1.2F) irrigation channel and a 1.2-mm (3.6F) working channel through which a probe for EHL and a stone extraction basket can be passed. Slightly larger miniscopes with bidirectional angulation systems and instrumental channels were developed by several companies. Despite these advances, POCS has remained a very cumbersome and time-consuming procedure that has not reached the expected popularity because it requires 2 experienced endoscopists to perform and has a small spectrum of possible applications. However, image quality and size have been significantly improved by the implementation of video cholangioscopes that use high-resolution video chips ( Fig. 1 ). The charge-coupled device (CCD) video chip is mounted in the distal tip of the scope and provides a 100° forward direction field of view. Disadvantages of these scopes were the lack of an accessory channel for interventional applications or a very limited working channel of only 0.5 mm. A newer “hybrid” video baby scope integrates a 1.2-mm accessory channel into a scope of 2.8 mm external diameter. The CCD unit is located in the control section, which protects the video chip from the usual scope trauma and may improve the durability of the scope. The image quality of glass fiber–based hybrid cholangioscopes with the CCD unit located in the control section is inferior to those with the CCD unit located in the tip of the scope. However, the video cholangioscope is still fragile and is not yet widely available. Despite technological advances in the design and maneuverability of miniature endoscopes and their accessories, few technical improvements have been made regarding the main concept of the mother-baby system. Limited tip deflection and the lack of optimal irrigation systems compromise visibility, requiring extra time and effort to complete the procedure. An additional limitation is the small caliber (range 0.5–1.2 mm) of the working channel that allows the use of only small biopsy forceps, which are able to obtain very small and often inadequate tissue samples. Table 1 summarizes and compares the currently available cholangioscopic technologies.
| Distal Diameter (mm) | Accessory Channel (mm) | Working Length (cm) | Angulation (up/down/left/right) | Field of View | Route | |
|---|---|---|---|---|---|---|
| Olympus | ||||||
| CHF-BP30 (fiberoptic) | 3.4 | 1.2 | 187 | 160°/130°/−/− | 90° | POCS |
| CHF-BP160 (video) | 2.9 | 0.5 | 200 | 90°/90°/−/− | 90° | POCS |
| CHF-BP160F (fiberoptic + video) | 2.8 | 1.2 | 200 | 70°/70°/−/− | 90° | POCS |
| CHF-CB30 (fiberoptic) | 2.7 | 1.2 | 45–70 | 120°/120°/−/− | 75° | PTCS |
| Pentax | ||||||
| FCP-8P (fiberoptic) | 2.8 | 0.75 | 190 | 90°/90°/−/− | 90° | POCS |
| FCP-9P (fiberoptic) | 3.1 | 1.2 | 190 | 90°/90°/−/− | 90° | POCS |
| FCN-15X (fiberoptic) | 4.8 | 2.2 | 35 | 180°/130°/−/− | 125° | PTCS |
| Boston Scientific | ||||||
| SpyGlass probe | 0.77 | 300 | 30°/30° | 70° | POCS | |
| SpyScope catheter (4 lm) | 3.3 (10F) | 1.2 + 0.6/0.6 | 220 | 30°/30° | ||
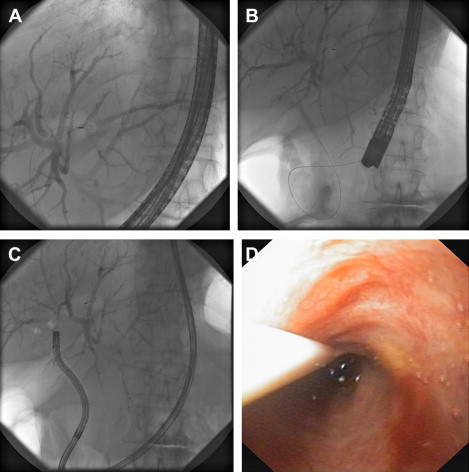
The search for a less cumbersome technique has led to the application of ultraslim gastroscopes to perform direct visualization and treatment of biliary disease. With external diameters of 5 to 6 mm, these instruments are only suitable for examination of the CBD after a sphincterotomy. An ERCP is performed first to place a 0.035-in diameter super-stiff guidewire as far into the bile duct as possible. After sphincterotomy the duodenoscope is cautiously removed and the ultraslim upper endoscope is then inserted over the guidewire, under fluoroscopic and endoscopic control, into the duodenum and across the ampulla of Vater into the CBD. Even with the guidewire in place, intubation and deep advancement of the endoscope into the bile duct can be limited by vector forces that tend to advance the scope along the axis of the duodenum, as well as looping the scope in the stomach. Loop formation can be reduced by use of an overtube, which can be mounted on the gastroscope, to facilitate this part of the procedure. Although the currently available overtube is too large in diameter for an ultraslim endoscope, making it difficult to manipulate both the overtube and the endoscope, it has been frequently adopted among Japanese and Korean endoscopists during POCS with ultraslim upper endoscopes. A pilot study evaluated the feasibility of POCS with an ultraslim endoscope and an intraductal balloon that can be anchored in a branch of an intrahepatic duct (IHD) with the similar procedure using a guidewire. A specialized 5F balloon catheter is required to fit the 2.0-mm working channel of the endoscope. After anchoring the intraductal balloon within a biliary branch, the endoscope can be advanced over the balloon catheter into the proximal biliary system. The investigators reported a success rate of 95.2% intraductal balloon-guided POCS compared with 45.5% for wire-guided POCS, and described no difficulties in negotiating through the hilum into the right or left ductal system once they were in the CBD. In addition, repeated advancement and withdrawal of the scope through the firmly anchored balloon catheter was possible. Nevertheless, anchoring the balloon within a branch of the IHD is not possible in some patients, and the intraductal balloon should be withdrawn from the scope to perform tissue sampling or therapeutic intervention, which can create technical difficulties in maintaining the desired endoscope position. An ultrathin balloon catheter was recently developed for placement over a guidewire by means of standard ERCP. After inflation and anchoring in the proximal biliary tree the balloon can be sealed before detachment of the handle. This technique allows removal of the duodenoscope and backloading of an ultraslim gastroscope for subsequent insertion over the anchored balloon catheter. The catheter can then be removed for insertion of accessories such as biopsy forceps ( Fig. 2 A–D). This promising new method is currently under clinical evaluation.
POCS with ultraslim endoscopes offers advantages such as performance by a single operator and superior image quality of the ductal mucosa. Furthermore, high-definition imaging and narrow-band imaging (NBI) can be obtained, which leads to a better visualization of neoplastic lesions. The separate water and air channels improve intraductal visualization and the 2.0-mm working channel allows the passage of larger biopsy forceps, which may increase the diagnostic yield during tissue sampling. Moreover, the working channel enables therapeutic interventions including EHL, LL, tissue ablation, and direct stent placement. Table 2 summarizes and compares the currently available ultraslim endoscopes used for cholangioscopy.
| Distal Diameter (mm) | Accessory Channel (mm) | Working Length (cm) | Angulation (up/down) | Angulation (left/right) | Field of View | |
|---|---|---|---|---|---|---|
| Olympus | ||||||
| GIF-N230 | 6 | 2 | 92.5 | 180°/180° | 160°/160° | 120° |
| GIF-XP 160 | 5.9 | 2 | 103 | 210°/90° | 100°/100° | 120° |
| GIF-XP 180 N | 5.5 | 2 | 110 | 210°/90° | 100°/100° | 120° |
| Pentax | ||||||
| FG-16 V | 5.3 | 2 | 92.5 | 180°/180° | 160°/160° | 125° |
| Fujinon | ||||||
| EG-530 N | 5.9 | 2 | 110 | 210°/90° | 100°/100° | 120° |
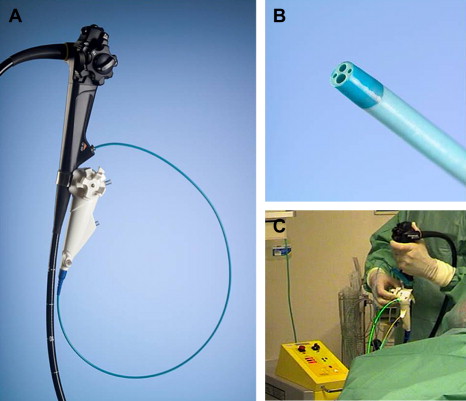
POCS with the mother-baby system can be performed by a single endoscopist with the help of a baby scope–holding breastplate. This technique has been reported to be successful in management of patients with choledocholithiasis. The recently introduced SpyGlass Direct Visualization System is designed for single-operator examination as well. This system can be strapped to the duodenoscope just below the operating channel with a silastic belt. The modular system consists of 3 components: (1) a reusable 0.77-mm diameter 6000-pixel fiber-optical probe (SpyGlass) for direct visual examination of the targeted bile duct; (2) a 10F disposable 4-lm catheter (SpyScope) consisting of a 0.9-mm channel for the optical probe, a 1.2-mm instrumentation channel, and 2 dedicated 0.6-mm irrigation channels; (3) a disposable 3F biopsy forceps (SpyBite) for tissue acquisition in the biliary system. The catheter has 4-way tip deflection (each more than 30°), which improves the maneuverability of the catheter in the duct and allows intubation of individual intrahepatic branches ( Fig. 3 A–C). An initial feasibility study demonstrated procedural success in 32 of 35 patients (91%) with indeterminate biliary strictures, with 71% sensitivity and 100% specificity in differentiating malignant versus benign pathologies and lesion-directed successful biopsy in 71% of patients. Furthermore, EHL performed under SpyGlass guidance cleared the bile duct from stones in all patients (5/5) after failure of prior conventional ERCP stone extraction. These results were confirmed by a prospective international multicenter registry reporting on 297 patients with either diagnostic or therapeutic indications in biliary disease. The study demonstrated an overall procedural success rate of 89% with a sensitivity of SpyGlass visual impression in diagnosing malignancy of 88%. Adequate stone visualization and initiation of stone fragmentation and removal was successful in 92%. Although the image quality is inferior to CCD chip cholangioscopes, visualization is enhanced by the 4-way deflected steering of the SpyGlass tip, which allows unrestricted access to all bile duct quadrants and the possibility of ample continuous irrigation to keep the field of view clear of blood, stone debris, sludge, or pus during visual inspection and biopsy. Therefore, direct visualization for the evaluation of biliary disease or therapy for biliary stones with the SpyGlass system can broaden the cholangioscopic options in diagnostic and therapeutic applications.
If the less invasive peroral route is not feasible or fails, PTCS can be performed by a single endoscopist along with an assistant for diagnostic and therapeutic interventions such as insertion of biopsy forceps or probes for lithotripsy. A sequential establishment of an appropriate cutaneobiliary fistula (“sinus tract”) is probably safer than a one-step approach. After MRCP-guided selection of the site of interest and selection of the most appropriate biliary segment, a percutaneous transhepatic catheter is placed. This tract can then be dilated every 2 or 3 days leaving larger drainage catheters in place up to a diameter of 16 French. This approach allows establishment of a mature tract within 8 to 10 days. A cholangioscope or bronchoscope can then be advanced into the biliary tree without need of a sheath. The tract predetermines the ducts that can be accessed with the cholangioscope; maneuvering to the opposite liver segment may be impossible through a single percutaneous tract. Cholangioscopes designed for percutaneous use have larger accessory channels and are accompanied by a broader array of therapeutic devices. The shorter working length and distance to the target area improve the ability to torque the scope for 4-quadrant visualization.
Diagnostic applications
The diagnostic indications for peroral cholangioscopy (POCS) including the evaluation of biliary strictures or intraductal filling defects are listed in Box 1 . PTCS is considered when transpapillary procedures fail because of inaccessibility of the papilla of Vater owing to previous surgery, difficult duodenal diverticulum, inaccessibility of a biliodigestive anastomosis, for example, hepaticojejunostomy, or nonpassable and not adequately dilatable bile duct strictures.
Visual characterization and optically guided biopsy of biliary strictures
Indeterminate strictures
Dominant strictures in primary sclerosing cholangitis (PSC)
Evaluation of fixed ductal filling defects noted on cholangiography or other imaging
Differentiation of benign versus malignant intraductal mass
Improved yield of tissue sampling under visual guidance
Enhanced image by use of chromocholangioscopy with dye solution, autofluorescence imaging (AFI), and NBI
Precise mapping and delineation of intraductal tumor spread before resection
Collection of significant fluid sample for cytology
Visual evaluation of intraductal spread of ampullary adenoma
Visual evaluation of choledochal cyst
Visual evaluation for ductal ischemia after liver transplant
Evaluation with visual examination and tissue sampling for infections
Traditionally, ERCP may be of assistance in characterizing strictures by providing tissue sampling; however, the low yield rates of ERCP-based methods for securing diagnosis of malignancy range from 35% to 70%. Direct visualization of the biliary ducts using cholangioscopy increases the ability to differentiate and diagnose lesions accurately in comparison with standard imaging or ERCP techniques. The combination of POCS and ERCP improved the sensitivity of diagnosing malignant lesions from 58% to 93%. In addition, POCS was useful in evaluating filling defects of uncertain etiology and was able to correctly diagnose malignant and benign lesions with an accuracy of 100%. By evaluating mucosal changes, presence of neovascularization, and patterns of luminal narrowing, it was determined that bile duct tumors demonstrate unique optical characteristics that could allow their differentiation. Another study demonstrated that cholangioscopy could potentially improve the diagnosis of cholangiocarcinoma by allowing the optical recognition of an irregularly dilated and tortuous vessel, the so-called tumor vessel. The presence of tumor vessels had a sensitivity of 61%, but combining the optical observation of tumor vessels with PTCS-guided biopsy resulted in a diagnosis of malignancy in 96% of the patients. The negative predictive value of tumor vessels on a basis of a 1-year follow-up was 100%. Cholangioscopy guides tissue sampling by assessing for tumor vessels, intraductal nodules or masses, infiltrative or ulcerated strictures, and papillary or villous mucosal projections. Prospective studies have shown that cholangioscopic visualization with and without biopsy has a sensitivity of 89% to 100% and specificity of 87% to 96%. PTCS-guided biopsy specimens obtained from bile duct carcinomas showed malignancy in 96%; however, the sensitivity of a single biopsy for the diagnosis of malignancy was only 62%, which demonstrated the requirement for multiple biopsies to obtain a higher diagnostic yield. In a cohort study sensitivities in detecting cholangiocarcinoma of patients with known cancer was 100% for the polypoid type, 95% for the stenotic type, and 100% when tumor vessel pattern was noted. Tissue sampling obtained from the margins and not from within strictures improved the histologic diagnosis rate of stenotic-type cholangiocarcinoma from 70% to 100%. A retrospective study described the use and yield of POCS, with and without mapping biopsies, to diagnose bile duct carcinoma and to assess the extent of proximal and distal intraepithelial tumor spread (ITS) to guide surgical resection. POCS improved the accuracy in the diagnosis of ITS from 79.5% to 100% and showed a diagnostic accuracy in assessing the extent of ITS of 76.9%, which was increased to 100% by adding mapping biopsies to POCS. Data for the semidisposable cholangioscope system have shown a sensitivity of 53% to 71% and a specificity of 82% to 100% in diagnosing malignancy using SpyGlass-directed biopsy. The 2.0-mm working channel of an ultraslim upper endoscope allows the passage of larger biopsy forceps, thus increasing the diagnostic yield during tissue sampling. With regard to diagnosing or refuting biliary malignancy and in assessing the extent of the disease, the future belongs to cholangioscopy. Brushings and cholangiographically guided biopsies, whatever additional techniques are applied, simply can never meet the critical precondition for optimal cytologic or histologic diagnosis, which is optimal tissue sampling under direct visual control. Future studies specifically designed to compare the diagnostic yield of endoscopic direct cholangioscopy and the semidisposable cholangioscope system with conventional cholangioscopy are pending. In terms of its sensitivity, specificity, accuracy, and positive and negative predictive value, POCS is significantly superior to ERCP in distinguishing between malignant and benign dominant bile duct stenosis in patients with primary sclerosing cholangitis (PSC).
Image-enhanced cholangioscopy techniques have been evaluated for use in biliary diseases, including chromocholangioscopy with dye solution, AFI, and NBI in addition to conventional white light illumination. Three studies evaluated the usefulness of POCS or PTCS in biliary tract lesions, using 0.1% methylene blue for better observation. The investigators described neoplastic findings showing irregular mucosa with inhomogeneous and intensively dark-blue staining patterns, whereas nonneoplastic findings had a smooth surface mucosa with homogeneous staining. Although chromocholangioscopy has some potential for enhancing visualization of bile duct lesions, the presence of mucus, exudate, bile, or contrast tends to obscure mucosal details and often interferes with the ability to achieve adequate tissue sampling. With autofluorescence cholangioscopy normal mucosa appeared green and neoplastic lesions change from green to dark green or black, owing to differences in the intensity of the autofluorescence. The diagnostic ability of PTCS without and with AFI has shown a sensitivity of 88% and 100%, specificity of 88% and 53%, and accuracy of 88% and 71%. However, the frequency of false-positive results increased with AFI. Nonneoplastic granular mucosa tended to appear slightly dark green and bile was recognized as dark-green fluid. Furthermore, during POCS bile often hindered the field of view because saline irrigation is difficult to achieve through a small working channel. NBI, which is based on the modification of spectral features with an optical color-separation filter narrowing the bandwidth of spectral transmittance, enhances visualization of fine surface mucosal structures and mucosal capillary microvessels compared with white light endoscopy. The pilot studies using NBI in the biliary system only involve a small number of cases. The delineation of the proximal and distal margins of biliary tract lesions and identification of vessels on the surface of the lesions were evaluated. Significantly better identification of the surface structure and tumor vessels by NBI than with conventional observation was reported ( Fig. 4 A, B). In particular, at the sites of superficial tumor spread, NBI tended to be better than conventional observation at detecting the lesion and delineating the margins. Technically challenging is the sufficient removal of abundant bile, mucin, and blood, especially because bile and blood both appear as dark red in the NBI mode. Another promising imaging modality to improve the preoperative diagnosis of biliary neoplasms is probe-based confocal laser endomicroscopy with confocal miniprobes further miniaturized to enable their use via the instrumentation channel of cholangioscopes. In 14 patients the presence of irregular vessels using confocal laser microscopy enabled prediction of neoplasia with an accuracy rate of 86%, sensitivity of 83%, and specificity of 88%; respective accuracy for standard histopathology was 79%. Preliminary clinical experience suggests that these innovative enhanced imaging cholangioscopy techniques may help to distinguish benign from malignant diseases, and highlight certain features such as mucosal structures and mucosal microvessels. Prospective randomized studies are required to confirm the diagnostic value of these advanced imaging technologies.










