One of the major proceedings in the field of gastrointestinal endoscopy has been the advent of molecular imaging, which possesses the potential to have a significant effect on the existing diagnostic and therapeutic paradigms. Molecular imaging encompasses different methods that enable the visualization of disease-specific morphologic or functional alterations of the mucosa based on the molecular signature of individual cells. This development has been made possible by advancements in basic science coupled with technological innovations in endoscopy, both facilitating the identification and characterization of mucosal lesions in vivo based on the lesions’ molecular composition rather than their morphologic structure alone. Novel studies based on fluorescent antibody imaging pave the road toward clinical translation and give hope for improved diagnosis and targeted therapies in gastrointestinal diseases.
In the past decades, enormous progress has been made in unraveling the pathogenesis of malignant and inflammatory disorders of the gastrointestinal tract. The endeavors taken by scientists are reflected by the successful identification of specific cellular proteins critically involved in the immunopathogenesis of these diseases. These proteins have a decisive effect on the pathologic signaling pathways that lead to uncontrolled cellular proliferation, migration, and aberrant invasion or heightened resistance to apoptosis. These insights in basic science have rapidly been transferred from the laboratory to clinical implementation.
The most prominent application to make clinical use of the molecular insights gained by scientific research is reflected by the evolving diagnostic possibilities in immunohistochemistry, which subsumes diverse methods aimed at recognizing antigens in situ by means of labeled antibodies. Immunohistochemistry is based on the detection of a target antigen in cell and tissue preparations through the initial binding reaction of specific antibodies against the corresponding antigen. To make this immune reaction visible, a fluorochrome is conjugated to the antibody, which in turn is activated through a light source using excitation wavelengths maximally absorbed by the fluorochrome, leading to an immunofluorescence emission that can be evaluated under the microscope. The most commonly used labels for fluorescent immunohistochemistry are fluorescein isothiocyanate emitting green fluorescence or rhodamine conjugates that emit orange to red fluorescence. Immunohistochemistry in tissue preparations has consequently been established in clinical practice regarding structural and functional imaging at the cellular level, as well as regarding diagnostic properties, prognostic evaluation, and even pretherapeutic assessments. This development is especially visible in the setting of gastrointestinal oncology in which tagged antibodies directed against tumor-specific antigens are routinely used in clinical practice to stage and grade malignant lesions. The importance of this field is further emphasized by the advent of novel therapeutic strategies based on molecular targeted therapies that are based on a sound pathophysiological rationale.
The necessity for a sustained therapeutic response, which can only be the result of a more comprehensive approach in targeting critical steps of signal transduction pathways, is best reflected by new therapies not only in oncology but also to a lesser extent in inflammatory conditions. Examples for these molecular targeted therapies are antibodies directed against the epidermal growth factor receptor (EGFR) in colorectal carcinoma or anti-tumor necrosis factor antibodies in inflammatory bowel diseases. In conjunction with these therapeutic concepts, the need for molecular imaging methods increases as well.
However, ex vivo histopathologic examination of tissue preparations only offers a momentary snapshot, reflecting the instance where the biopsy specimen is taken from the mucosa, thereby negating dynamic processes taking place in the tissue. Furthermore, the antigen is removed from its natural surroundings and its immunologic activity could therefore be significantly altered. The various fixation and staining processes, which the mucosal specimen is subjected to while the histopathologic section is made, represent another source that could severely influence the expression of the examined antigen. Besides these limitations, the possible risk of physical bleeding and other complications while taking a mucosal sample give reason for the need of alternative methods for nondestructive in situ molecular imaging.
Various imaging modalities have subsequently been used in the past years in different preclinical or clinical settings to evaluate molecular processes in vivo and have given the possibility to analyze the feasibility of various approaches.
Positron emission tomography (PET) and single-photon emission computed tomography (SPECT) have shown considerable promise in this regard and have been used for human molecular imaging for many decades now. The use of PET radiotracers allows the imaging of intracellular molecular processes known to be present during malignancy and thereby enables the localization of primary tumors and metastatic foci in the gastrointestinal tract. PET radiotracers can also assess the efficacy of targeted therapies on a molecular level. This response assessment is therefore beyond the mere evaluation of the size of the tumor because it also takes into account potentially relevant molecular effects, such as anti-angiogenesis, that occur in the initial phase of the treatment. This development is reflected by the introduction of novel radiotracers, which analyze the molecular effects of targeted oncological therapy. A well-established method is the use of fludeoxyglucose F 18 that images glucose metabolism to detect gastrointestinal cancers during PET examinations. The other marker under investigation is 18 F 3-deoxy-3-fluorothymidine as a marker for cell proliferation, which might be used in the assessment of early response to treatment. There have also been other reports concerning the use of these imaging modalities for the assessment of response to specific therapies. By using technetium Tc 99m annexin V SPECT to visualize the rate of apoptotic intestinal cells in vivo, it was recently demonstrated that treatment with the anti-tumor necrosis factor antibody infliximab induces apoptosis in lamina propria mononuclear cells in patients with active Crohn disease. Moreover, the induction of apoptosis in intestinal cells also correlated with the clinical efficacy of this treatment.
Nevertheless, there is still the need for the introduction of optical molecular imaging in endoscopic procedures, because only this examination provides real-time image information in vivo coupled with the possibility to intervene in the gastrointestinal tract during the identification of the mucosal lesion and can thus be used instantly for molecular targeted procedure guidance. Advances in optical devices, refined biologically derived materials, innovative fluorochromes- and novel conjugation techniques contribute to the recent advances made in this field of molecular imaging and give reasonable hope for improved diagnosis and targeted therapies in gastrointestinal diseases. The following sections provide an overview of the diagnostic and therapeutic potential of optical molecular imaging in the gastrointestinal tract.
Molecular imaging in gastrointestinal endoscopy
Instruments for Molecular Imaging
During the last few years there have been tremendous advancements in optical and mechanical technologies for imaging in the gastrointestinal tract. Since the implementation of flexible fiberoptics in endoscopic devices, gastrointestinal imaging has advanced to include video imaging and lately, high-definition systems. Further developments led to image-enhancing methods such as chromoendoscopy, which depicts the topical application of different intravital dyes (methylene blue, indigo carmine) onto the mucosal surface to contrast pathologic lesions against the normal mucosa. The introduction of virtual chromoendoscopy, such as narrow band imaging, or other surface-enhancement modalities improved this development by increasing the contrast between normal and altered tissue.
Although these innovations greatly improved endoscopic possibilities, several studies have shown that in screening and surveillance examinations of the gastrointestinal tract there is still a significant rate of undetected pathologic lesions in the mucosa. Therefore, molecular imaging in the context of endoscopic examinations of the gastrointestinal tract aims at the identification and characterization of mucosal lesions in vivo based on the lesions’ molecular composition rather than their morphologic structure alone. Further initiatives to increase the optical contrast in endoscopic examinations are based on using endogenous fluorescence or exogenously applied fluorochromes.
Autofluorescence imaging uses the effect that a tissue that is exposed to light of a defined wavelength responds by emission of light of a longer wavelength because of the excitation of endogenous fluorophores. Dysplastic tissue has an altered composition of endogenous fluorophores and therefore- exhibits a changed autofluorescence spectrum resulting in false-colored images that distinguish themselves from the background of the healthy mucosa. Nevertheless, recent trials showed that this method was not able to significantly improve the diagnostic outcome in the detection of mucosal pathologies, such as adenoma. Contrastingly, another study found a lower neoplasia miss rate in patients with ulcerative colitis compared with standard white light endoscopy. Further studies are required to fully evaluate this technique in screening endoscopies. The limitation of this endoscopic procedure however, is its low specificity and therefore the high false-positive rate in the detection of mucosal lesions. Tissue alterations are not specific for certain pathologies, and inflamed mucosa severely affects the specificity of depicting neoplastic changes.
Further technological advances are reflected by various means of increased magnification of the tissue during endoscopic procedures. This development has culminated in the emergence of confocal laser endomicroscopy (CLE), which is a powerful tool for performing real-time in vivo imaging in the mucosal tissue at the cellular and subcellular levels. This endoscopic technique not only incorporates the benefits of high-resolution imaging of confocal microscopy but also enables subsequent in situ immunofluorescence staining or other fluorescent labeling techniques. At present, 2 CLE-based systems are available, consisting of an integrated endoscopy system and a probe-based system. The integrated system consists of a conventional white light endoscope in which a confocal fluorescence microscope has been integrated into the distal tip. The system uses a 488-nm wavelength laser and enables the detection of fluorescence between 205- and 585-nm wavelengths. The variable imaging depth ranges from the surface to 250 μm.
The flexible probe-based systems are compatible with the working channels of the different endoscopic devices and use blue laser excitation and fluorescence detection over 505 nm. All the commercially available probes have varying imaging planes, which are mostly fixed in each probe and cannot be adapted. The range of these planes lies between 40 and 350 μm. CLE mandates the use of fluorescent agents to enhance contrast, and most of the trials performed so far have successfully used intravenous fluorescein sodium to enhance contrast.
Imaging Agents
The choice of the appropriate imaging agent is based on its molecular size, stability, safety profile, and the specific spectral characteristics of the fluorochrome. Fluorescein and indocyanine green have both been approved for use in humans and exhibit different emission wavelengths. Whereas fluorescein emits green fluorescence, indocyanine green is characterized by near-infrared fluorescence. Indocyanine green has only limited use in molecular imaging because it is difficult to be conjugated with other substances. Therefore, fluorescein is clearly the most widely used fluorescent agent because it can easily be attached to various protein structures. The emission wavelengths of fluorescein are set in the range of 520 to 530 nm during stimulation by lasers at wavelengths of 465 to 490 nm. Adverse events caused by fluorescein usage in humans are rare, and the overall safety profile of this substance in the numerous studies performed so far has been convincing.
Route of Application
There are 2 primary possible routes for the application of the agent for optical molecular imaging. Topical spraying of the substance onto the mucosal surface has been the most widely used method so far because it has many advantages compared to intravenous administration. The relatively large size of the molecular agent used usually prevents it from being able to pass through the epithelial barrier, and thereby, the exposition of the patient to systemic concentrations of the imaging agent is relatively low. Thus, topical application obviously greatly minimizes the risk of possible adverse events and side effects in comparison to intravenous administration. On the other side, there is still the risk for the occurrence of local reactions of the mucosa to the imaging agent, and therefore, the examined area has to be closely monitored for some time after topical application has taken place. Topical application is more feasible because the agent can normally be applied during or right before the imaging procedure, whereas intravenous application requires a preliminary lead time for the agent to get distributed throughout the body has taken place. Topical application of the agent, usually administered via a standard spraying catheter, is not reasonable for use in large mucosal surfaces but is efficient if a region of interest has been identified before, where the agent can be specifically be applied to. Topical administration of the agent requires the target to be expressed on the luminal surface of the tissue or at least to be rapidly accessible by the agent to ensure that the necessary binding reaction takes place. Intravenous administration seems favorable if subsurface target structures have to be reached and an even distribution throughout the body is intended. Topical application seems to be advantageous concerning safety issues and if the target structure is expressed on the mucosal surface. Nevertheless, there are no molecular substances that have been approved for in vivo use in gastrointestinal endoscopy at present. Because the usage of radiolabeled probes for molecular imaging has become an integral diagnostic part in nuclear medicine, similar developments seem imaginable for endoscopic procedures as well, and current research activities are centered on the development of fluorescent-labeled probes for this setting.
Classes of Targeting Ligands
There are different groups of targeting ligands that are generally possible for molecular imaging modalities. The choice of the appropriate molecular probe depends on the target structure, the specificity of its signal, and its safety profile.
Antibodies are the obvious choice because they bind to their corresponding target structure in a highly selective manner and can be specifically developed against the epitopes of the antigen of interest. Antibodies are well established in the diagnostic field and labeling procedures with fluorescent agents, which are a prerequisite for molecular imaging and a common practice in in vivo preclinical imaging. Moreover, novel treatment regimens with monoclonal antibodies have evolved around the basic concept of targeting specific molecules that have a pivotal pathogenic role in the disease. Consequently, monoclonal antibodies have proved their clinical efficacy for a steadily increasing number of indications and thus have become a major asset in the therapeutic regime of various diseases. Because the biologic relevance of these targets has already been established, monoclonal antibodies already approved for therapeutic use represent an attractive targeting ligand in molecular imaging. It is conceivable that the intensity of the binding reaction of the antibody to the mucosal lesion could potentially predict the response to this therapy. In this case, the individual “antibody against the molecular target” binding saturation would directly correlate with the therapeutic efficacy of the substance. This approach is especially attractive in the prediction of response to targeted chemotherapy for malignant disorders and could therefore have direct therapeutic consequences, because it enables the stratification of patients before the initiation of the intended therapy. The high binding affinity for the defined target is another advantage of antibodies in molecular imaging because it minimizes the unspecific background signal. On the other hand, most of the used antibodies have a certain immunogenic property that may confer allergic reactions, especially after systemic application. The long half-life of these antibodies in blood may have an effect on the specificity of the signal, because systemic application could lead to the accumulation of the labeled antibodies and hence a heightened unspecific background signal. The high molecular weight and size of labeled antibodies lead to a slow and restricted delivery to the intended target structure, limiting its usage in systemic applications. Therefore, labeled antibodies are probably best used via topical application for the visualization of extracellular molecular targets expressed on the cellular surface.
Several novel approaches aim at formulating molecular probe classes that might be able to overcome the limitations of molecular imaging via labeled antibodies. The generation of antibody fragments has led to a significant reduction in the ligand size, resulting in improved clearance times while retaining high binding affinity to the target structure. Targeting peptides are a further development in molecular imaging because they consist of only few amino acids that have high target affinity and specificity, with even shorter blood clearance times. These peptides therefore have low immunogenic properties and seem suitable for topical administration in molecular imaging procedures. The so-called small molecules that are loaded with various proteins for stronger target recognition are other possible alternatives because they exhibit high specificity and could even be able to visualize intracellular targets or different targets with the same molecule. Their small size might enable them to penetrate more easily into diseased mucosa, thus potentially binding to molecular targets at greater tissue depths. However the conjugation of these small molecules with adequate fluorochromes increases its total size and could affect the biodistribution of the labeled molecule. Potential toxicologic effects of nonbiocompatible structures of these small molecules are further hindrances that have to be taken into consideration before clinically testing these promising substances for molecular imaging in endoscopy.
All these molecular probes have in common that their mode of action is based on direct binding to the target. This action is associated with the possibility of high background signals caused by unspecific binding that does not reflect the true level of expression of the biologic target. In contrast, refined classes of optical imaging agents have been newly developed, which change their fluorescent properties only after prior target interaction. These smart probes are initially in an optical non-activated state because the close proximity of the fluorochromes on the molecular probe results in autoquenching where almost no fluorescent signal can be detected. After binding to the target structure, the probes are activated by proteases overexpressed at the target site. The ensuing enzymatic cleavage of the fluorochromes results in activation and thus amplification of the fluorescent signal. This activation greatly enhances the signal to background ratio for fluorescent imaging and allows rapid screening of large surface areas. This approach has shown particular promise in preclinical studies exploiting the protease overexpression seen in experimental intestinal adenomas and adenocarcinomas. Another approach in this context are pH-activable probes, which are internalized after binding to target structures on the cell surface of malignant cells and are subsequently activated according to the prevalent pH. Again, the currently undefined safety profile has to be closely evaluated before clinical studies on these substances can be performed.
Molecular Targets
Based on novel findings derived from research endeavors unraveling the pathogenesis of inflammatory or malignant conditions, several biologic targets have been identified that come into consideration for molecular imaging in gastrointestinal endoscopy. The selected target has to be accessible for the molecular probe applied and specific for the gastrointestinal disorder investigated. In this setting, the possible biologic target structures are specific cell surface receptors, proteins in the extracellular matrix, various metabolites, intercellular or vessel structures and even distinctive subsets of cells. The decision which molecular structure or pathway should be targeted in molecular imaging procedures depends on the clinical setting in which the endoscopic examination takes place. Molecular imaging can be used for improved detection of polyps or adenocarcinomas in surveillance endoscopies to increase the contrast between normal and altered tissue. The sensitivity of the chosen target is essential for differentiating malignantly transformed and normal tissue. In situ characterization of already identified mucosal lesions, to determine if the finding is clinically significant and needs immediate handling or not, highly influences the selection of the molecular target because a higher specificity of the procedure is prerequisite.
Another highly attractive application for molecular imaging in gastrointestinal endoscopy is the possible stratification of patients to respond to molecular specific therapies before the initiation of the treatment. Reliable prediction of response to biologic therapies would enable a better selection of suitable patients for treatment beforehand, thus decreasing morbidity in patients with a low likelihood of response and enhancing cost-effective use of these treatment options. This approach assumes that there is a direct correlation between the expression levels of target molecules and the response to the associated biologic therapy directed against it. In this setting, basic science is indispensable because it is able to unravel the molecular mechanism of action of novel therapies to present possible molecular targets for imaging. Another likely approach is to label already approved biologic therapies with appropriate fluorochromes and subsequently quantify their binding reaction to the tissue, which might correlate with the specific therapeutic response. Appropriate selection of proliferation markers in malignant disorders of the gastrointestinal tract could also serve as molecular targets in endoscopic examinations to assess the efficacy of therapeutic strategies at an earlier stage than traditional anatomic imaging used at present.
Clinical studies using molecular imaging in gastrointestinal endoscopy
In vivo molecular imaging has been the subject of an increasing number of preclinical and clinical trials, with the potential to significantly affect basic and clinical science in the field of gastrointestinal endoscopy. In a pilot trial, a fluorescent-labeled monoclonal antibody directed against the carcinoembryonic antigen was topically applied during colonoscopy in patients with colorectal polyps or tumors. The endoscopic procedure was performed using a conventional endoscope in which the optical range was increased via narrow band filters. It was shown that a specific fluorescence signal was present in most carcinomas. This effort served as a valuable proof-of-principle study and enabled further trials.
Further advances were achieved in subsequent animal studies. In an important study, a substrate probe specific for the enzyme cathepsin B, a protease that is known to be upregulated in gastrointestinal tumors, was created and conjugated with a near-infrared–based signal that is quenched in its intact state. After intravenous administration of this molecular probe in mice, the cathepsin B–activated beacon demonstrated a high specificity in detecting even small adenomas during colonoscopy. In another study, this concept was taken even further toward a possible clinical application, when a commercially available capsule was equipped with a filter device capable of near-infrared imaging. In ex vivo studies, this capsule successfully detected and transmitted cathepsin B–derived near-infrared fluorescence signals in mouse models for intestinal polyposis and inflammation. These works demonstrated the possibilities of molecular imaging by capsule endoscopy, and further studies are awaited.
The introduction of confocal endomicroscopy has led to further developments in molecular imaging. In a melanoma mouse model, in vivo confocal imaging with a handheld probe enabled the visualization of tumor cells after the injection of fluorescein-labeled antibodies against octreotate.
In a landmark trial, neoplastic lesions were detected during colonoscopy, using a labeled heptapeptide derived from a phage library. Through the use of a probe-based endomicroscopic system, it could be shown that the topically applied fluorescein-conjugated peptide did preferentially bind to neoplastic cells with a sensitivity and specificity greater than 80%. In a further study, a fluorescent-labeled antibody targeting the EGFR was tested against human xenograft tumors in mice. In this experimental setting, confocal endomicroscopy could accurately identify EGFR expression. In human tissue samples ex vivo, confocal endomicroscopy was even able to differentiate between malignantly transformed tissue and non-neoplastic tissue after topical application of the fluorescent-labeled antibody.
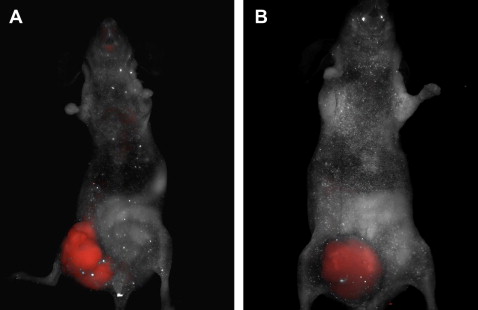
Own studies used the concept of molecular imaging to visualize the processes of tumor angiogenesis in a xenograft mouse model ( Fig. 1 ). Because the process of neovascularization has long been identified as an essential step in promoting tumor growth and dissemination, modulation of angiogenesis is currently considered to be one of the most promising therapeutic strategies in patients with advanced colorectal cancer (CRC). Studies on CRC have focused on integrins as targets for pharmaceutical intervention because these adhesion molecules play a major role in regulating cell adhesion to the extracellular matrix and are also directly involved in carcinogenesis and metastasis. The integrin α v β 3 is expressed by newly formed blood vessels in diseased and neoplastic tissue and therefore represents a reliable marker for angiogenesis in CRC. It could be shown that the vascular expression level of α v β 3 in colon carcinomas has a high prognostic value for overall survival. Therefore, evaluation of α v β 3 levels in CRC could serve as a valuable indicator for the course of the disease and may also have direct implications for the assessment of response to anti-angiogenic therapies. Hence, molecular imaging modalities represent an attractive tool for targeted visualization of this tumor-associated molecule in vivo. Therefore, in initial studies, the authors injected SW620 CRC cells into nude mice. After intestinal tumor formation, fluorescent markers for the integrin α v β 3 were administered intravenously into the xenograft mice model. Targeted fluorescence signals for α v β 3 were visualized in vivo via a full body fluorescent scanner, demonstrating increased angiogenesis in the cancer tissue (see Fig. 1 A). The vascular endothelial growth factor (VEGF) is another important molecule promoting tumor growth and vascularization in neoplastic intestinal tissue. Subsequently, a monoclonal antibody against VEGF (bevacizumab) was the first anti-angiogenic drug approved for the treatment of metastatic CRC. One of the corresponding receptors for VEGF is the vascular endothelial growth factor receptor 2 (VEGFR-2), which seems to mediate almost all of the known cellular responses to VEGF. Again, in vivo macroscopic imaging was performed in the xenograft tumor mouse model using a fluorescent-labeled antibody against the VEGFR-2 and a multispectral in vivo imaging system (see Fig. 1 B). Strong signal intensity was observed specifically at the site of the tumor. Foersch and colleagues proved that the targeted visualization of a tumor-associated molecule can similarly be achieved via CLE in fresh tumor specimens of human CRC. After topical application of fluorescent-labeled antibodies against the VEGFR-2, CLE displayed sufficient signal intensity and an adequate contrast in the examined neoplastic tissues ( Fig. 2 ). The feasibility of this method indicates that similar data might be obtained in the future while performing CLE in vivo.