Nanomedicine is a new distinct scientific discipline that explores applications of nanoscale materials for various biomedical applications. Translational nanomedicine is undergoing rapid transition from development and evaluation in laboratory animals to clinical practices. In the future, it is anticipated that nanotechnology can provide urologists a new point of view to understand the mechanism of disease, tools for early diagnosis of the disease, and effective modality for treatment. This article summarizes some of the emerging applications of nanomedicine in urology.
Nanomedicine is a new distinct scientific discipline that explores applications of nanoscale materials (1–1000 nm) for various biomedical applications. At nanoscale, the physical properties of materials are altered, as are their interactions with cells and tissue. This occurs primarily because of the significant difference in the surface area/volume ratio as materials are reduced to nanosized level. Nanomedicine explores nanotechnology for monitoring, repair, and control of human biologic systems at cellular and molecular levels using engineered nanodevices and nanostructures. Nanomedicine can improve diagnosis and treatment, and it can also be used in tissue engineering to replace some functions of human organs. The potential scope of nanotechnology in urology is wide-ranging, from prevention to early detection, treatment, prognosis, and symptom management. The most common nanoscale drug delivery and imaging vehicles (nanocarriers) include polymeric nanoparticle, dendrimers, nanoshells, liposomes, nucleic acid–based nanoparticles, magnetic nanoparticles, and virus nanoparticles. This article summarizes some of the emerging applications of nanomedicine in urology.
Application of nanotechnology for diagnosis
Application of Nanotechnology for Imaging
Applications of nanotechnology in the diagnosis of genitourinary diseases have been extensively studied in recent years. Advances in nanotechnology have shown the promise of nanoparticles for noninvasive tumor imaging. Noninvasive imaging approaches, such as X-ray–based CT, positron emission tomography, single-photon emission CT, and MRI, are used as important tools for detection of human cancers. The development of tumor-targeted contrast agents based on nanoparticle formulations has been shown to increase the sensitivity and specificity of tumor imaging using currently available imaging modalities. Superparamagnetic iron oxide or iron oxide nanoparticles are becoming increasingly attractive as the precursor for the development of target-specific MRI agents. Magnetic nanoparticles can shorten both T1 and T2 and enhance MRI contrast. T1 shortening processes require a close interaction between protons and T1 agents, however, which can be hindered by the thickness of the coating on the magnetic nanoparticles. The T2 shortening is caused by the large susceptibility difference between the particles and surrounding medium.
Magnetic nanoparticles can extravasate into the interstitial space and subsequently transport to lymph nodes where they are taken up by macrophages. This can be used to detect lymph node metastasis. Within the lymph nodes, lymphotropic superparamagnetic nanoparticles are internalized by macrophages and change magnetic properties detectable by MRI. Renal carcinoma is the third most common genitourinary tumor. The lymphatic metastasis is associated with the patient’s survival; it is important to improve the detection of nodal metastases. MRI is widely used to diagnose renal cancer. Although it provides images with excellent anatomic details and soft tissue contrast, it is relatively insensitive to detect lymph node metastases. Lymphotrophic nanoparticle-enhanced MRI (LNMRI) has been used to identify malignant nodal involvement in patients with renal neoplasm. In one study, monocrystalline iron oxide magnetic nanoparticles were administrated by intravenous method, and imaging was performed before, immediately after, and 24 hours after administration. LNMRI showed high sensitivity (100%) and specificity of detection of lymph note metastasis (95.7%).
Prostate cancer is one of the most common cancers in North America. After the diagnosis of prostate cancer, accurate detection of lymph node metastases is essential to determine the extent of the disease to select appropriate therapy. Patients with local prostate cancer can receive radical prostatectomy, watchful waiting, or radiotherapy; however, patients with advanced or metastatic prostate cancer require an adjuvant androgen-deprivation therapy. Nanoparticles are used to detect the nodal metastases in patients with prostate cancer. In one study, all patients with nodal metastases were identified by MRI with lymphotropic superparamagnetic nanoparticles, and node-by-node analysis showed MRI with nanoparticles had a significantly higher sensitivity than conventional method (90.5% versus 35.4%; P <.001). LNMRI is also used to detect the metastases within retroperitoneal nodes in patients with testicular cancer; the sensitivity, specificity, and accuracy of LNMRI for malignant lymph node involvement were 88.2%, 92%, and 90.4%, respectively. The sensitivity, specificity, and accuracy of size criteria for detecting malignant nodes were 70.5%, 68%, 69%, respectively. These clinical trials suggest a promising future of applications of LNMRI in urology.
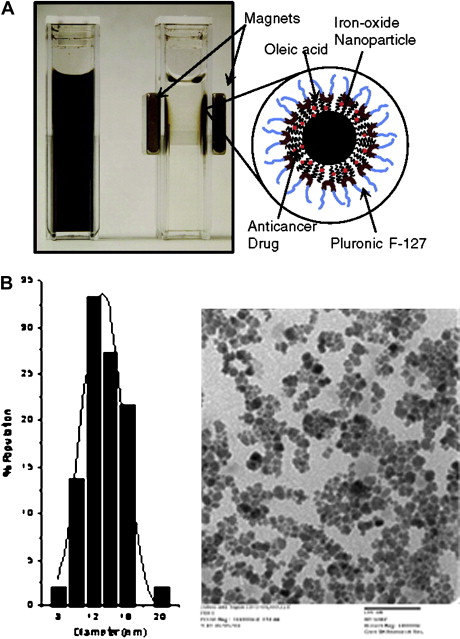
Recently, Jain and colleagues have developed biocompatible magnetic nanoparticles with dual functional properties. The iron-oxide core is first coated with oleic acid and then oleic acid–coated particles are stabilized with plutonic F127 to make them dispersible in aqueous vehicle. Plutonic prevents protein binding and particles aggregation, which reduces their rapid clearance by the reticuloendothelial system, keeping nanoparticles in systemic circulation to allow their extravasation into tumor tissue. Doxorubicin (base form) and paclitaxel were shown to load these nanoparticles with high efficiency (75%–95%), and the loaded drugs are released over 2 weeks, sustaining the drug effect. Further, a combination of drugs can be loaded in these nanoparticles for synergistic activity. These multifunctional nanoparticles have greater advantage as compared with conventional magnetic nanoparticles because they can be used for both drug delivery and imaging applications ( Fig. 1 ).
Semiconductor quantum dots are nanometer-scale, light-emitting particles, which have some unique optical and electronic properties, such as size-tunable light emission, improved signal brightness, enhanced stability of the fluorescent signal, and the ability to simultaneously excite multiple fluorescent colors. These characteristics allow quantum dots to have broad absorption, narrow and symmetric emission spectra, long-term photostability, and continuous absorption spectra, and they are easy to use as probes for multicolor imaging compared with conventional dyes. Gao and colleagues developed a bioconjugated quantum dot probe suitable for in vivo targeting and imaging in mice. The conjugated quantum dots are modified with an amphiphilic tribolck copolymer for in vivo protection, antibody to target prostate-specific membrane antigen (PSMA), and multiple polyethylene glycol (PEG) molecules to improve biocompatibility and circulation. Imaging study in vivo demonstrated that quantum dots probes can be targeted to prostate tumor sites in mice through both passive and active mechanism, but passive targeting is much slower and less efficient than active targeting.
Application of Nanotechnology for Detection of Single Nucleotide Polymorphism
Progress in nanotechnology allowed detection of single-nucleotide polymorphisms in genes related to cancer, genetic disease, and nitrification. Autosomal-dominant polycystic kidney disease is a genetic disease of humans. Autosomal-dominant polycystic kidney disease is characterized by enlarged polycystic kidneys and results in end-stage renal disease. Autosomal-dominant polycystic kidney disease is caused by mutations of two genes: PKD1 and PKD2. Son and colleagues have developed a rapid, accurate, and inexpensive nanoparticle-DNA–based assay to detect PKD single-nucleotide polymorphism mutations in hybridizations-in-solution platform. The Fe3O4/Eu:Gd2O3 and Fe3O4/Tb:Gd2O3 core-shell nanoparticles were used to capsulate DNA. The PKD single-nucleotide polymorphisms from kidney tissue and blood samples can be detected without a polymerase chain reaction step, which is convenient. The sensitivity of this method is very high and for blood genomic DNA, only 0.02 to 0.05 mL of whole blood sample is needed for detection.
Application of Nanosensor for Bacterial Detection
Basu and colleagues developed a quick and sensitive procedure for bacterial detection in case of kidney infection. The procedure is based on both optical and electrochemical studies. Detection method used gold nanowire devices in conjunction with a linker arm attached to specific Escherichia coli antibodies. The study showed that the biosensor can detect each of 50 E coli cells with the sensor area of 0.178 cm.
Application of nanotechnology for treatment
Nanocarrier Delivery of Drugs for Treatment of Urologic Cancers
Nanoscale vehicles have been extensively investigated to delivery anticancer drugs. The most common examples of the nanoscale delivery vehicles include polymeric nanoparticles, dendrimers, nanoshells, liposomes, nucleic acid–based nanoparticles, magnetic nanoparticles, and virus nanoparticles. Current chemotherapeutic drugs not only kill cancer cells, but also healthy cells and cause significant toxicity to patients. The nanocarrier-based delivery of anticancer drugs to tumor tissue can be achieved by either passive or active targeting; hence, these methods of drug delivery can increase the effect of drug while reducing side effects. Tumors tissue has leaky blood vessels and poor lymphatic drainage. Although free drugs may diffuse nonspecifically, a nanocarrier can extravasate into the tumor tissue by way of the leaky vessels by the enhanced permeability and retention. The dysfunctional lymphatic drainage in tumor facilitates nanocarriers to accumulate in tumor tissue and release drugs into the vicinity of the tumor cells. Active targeting of tumor cells is achieved by conjugating nanocarriers containing chemotherapeutics with molecules that bind to overexpressed antigens or receptors on the target cell.
Drug resistance is one of the major obstacles limiting the therapeutic efficiency of chemotherapeutic or biologic agents. The mechanism of cancer drug resistance is complex. More often, it is caused by the overexpression of multidrug drug resistance transporters; the transporters actively pump chemotherapeutic drugs out of the cell and reduce the intracellular drug dose below lethal threshold levels. Nanocarriers can bypass the multidrug drug resistance by preventing anticancer drugs from encountering the transporters. Sahoo and Labhasetwar studied cytotoxicity of transferrin-conjugated and unconjugated paclitaxel-loaded nanoparticles in vitro in drug-resistant cell lines. They found the conjugated nanoparticle can overcome drug resistance by sustaining intracellular drug retention.
7-Ethyl-10-hydroxy-camptothecin (SN-38) is a biologically active metabolite of irinotecan hydrochloride (CPT-11) and has potent antitumor activity. Sumitomo and colleagues used SN38-incorporated polymeric micelles, NK012, to treat the renal cell carcinoma model established by inoculating murine Renca cells and human renal cancer cells SKP-9. Compared with CPT-11, NK012 was shown to have significantly higher antitumor activity against both bulky Renca tumors and SKRC-49 tumors than drug alone. In the pulmonary metastasis model, administration of NK012 enhanced and prolonged distribution of free SN-38 in metastatic lung tissues; the concentration of SN-38 in nonmetastatic lung tissues was much lower. NK012 treatment decreased the metastatic nodule number significantly. These results demonstrate the significant advantages of polymeric micelle-based drug carriers and the authors suggested that NK012 would be effective in treating disseminated renal cancer with irregular vascular architectures.
Current treatment of superficial bladder cancer consists of transurethral tumor resection and chemotherapy. Chemotherapy usually follows surgery to reduce tumor recurrence or progression. Intravesical chemotherapy can selectively deliver drugs to bladder while minimizing systemic exposure. However, the response of intravesical chemotherapy is incomplete, and variable among patients; this is partly caused by the inability of drug to penetrate bladder tissue. Chemotherapeutic drugs loaded with nanocarriers provide more efficient and specific approaches to treat bladder cancer than drug alone. Paclitaxel for clinical use is dissolved in polyoxyl compound; however, it reduces the free fraction of paclitaxel and consequently lowers the drug penetration into the bladder tissue. Lu and colleagues developed paclitaxel-loaded gelatin nanoparticles for intravesical delivery to increase the penetration of paclitaxel into bladder tissue. The paclitaxel-loaded gelatin nanoparticles can release the drug rapidly, resulting in much higher drug concentrations in the urothelium and lamina propria than paclitaxel in Cremophor formulation. Bladder transitional cell carcinoma overexpresses the transferrin receptors on the surface of cells. Derycke and colleagues examined penetration and accumulation of transferrin-mediated liposomal targeting of the photosensitizer, aluminum phthalocyanine tetrasulfonate (AlPcS4), in bladder tumor. AlPcS4 was encapsulated in unconjugated liposomes (Lip-AlPcS4) or transferrin-conjugated liposomes (Tf-Lip–AlPcS4). The accumulation of free AlPcS4, Lip-AlPcS4, and Tf-Lip–AlPcS4 in human AY-27 transitional-cell carcinoma cells and in an orthotopic rat bladder tumor model was visualized by fluorescence microscopy. Results showed accumulation of Tf-Lip–AlPcS4 was much more than that of Lip-AlPcS4 (384.1 versus 3.7μM; P = .0095). The in vivo study showed that intravesical instillation of Tf-Lip–AlPcS4 resulted in specific accumulation of AlPcS4 in tumor tissue, instillation of free AlPcS4 resulted in nonselective accumulation throughout the whole bladder wall, and instillation of Lip-AlPcS4 resulted in no tissue accumulation. Photodynamic therapy of AY-27 cells showed Tf-Lip–AlPcS4 had high cytotoxicity. The results suggested that transferrin-mediated liposomal targeting of photosensitizing drugs is a promising potential tool for photodynamic therapy of superficial bladder tumors. Submucosal injection of doxorubicin-loaded liposome in bladder was shown to result in better distribution of drug and prolonged retention through the bladder wall and regional lymphoid nodes compared with free drug. The result suggests the therapeutic use of nanocarrier not only in superficial bladder cancer but also in invasive bladder cancer and regional lymphoid node metastasis.
Because of its high mobility, prostate cancer always causes great interest to researchers. There are several examples of applications of chemotherapeutic-drug loaded nanocarriers for treating prostate cancer. Doxorubicin-loaded micelle and curcumin-loaded liposomes have been shown to improve the efficacy over free drugs. It has been shown that A10 2′-fluoropyrimidine RNA aptamer (Apt) can bind to the PSMA. A combination of Apt and antibody against PSMA (J591) conjugated to nanoparticles was shown significantly to improve the uptake of nanoparticles by PSMA (+) prostate cancer cells ( Fig. 2 ). In another approach, the fact that transferrin receptors are overexpressed on the surface of prostate cancer cells was used to improve drug delivery. Sahoo and colleagues used transferring-conjugated sustained-release paclitaxel-loaded biodegradable nanoparticles to treat prostate cancer. The in vivo experiment in subcutaneous animal model by direct intratumoral injection of transferrin-conjugated nanoparticles showed complete tumor regression compared with paclitaxel–polyoxyl compound and paclitaxel-loaded nanoparticle without transferrin ( Fig. 3 ). The efficacy of transferrin-conjugated nanoparticles is suggested to be caused by enhanced cellular drug uptake and sustained drug retention in tumor tissue. Direct intratumoral injection of drug-loaded nanoparticles can be effective in the treatment of localized tumor, such as prostate or kidney tumors, and could be a preferred option over surgical intervention to remove tumor.
The possibility of thermal therapy of prostate cancer with nanocarriers is another interesting approach, particularly to treat cancer refractory to chemotherapy. Kawai and colleagues examined the hyperthermic effect of magnetic particles in rat prostate cancer. Magnetic liposomes generate heat in an alternating magnetic field. The tumor temperature can increase to 45°C, whereas the body temperature remains at around 38°C. Significant tumor regression was observed in the hyperthermic group. Immunohistochemical staining showed the presence of CD3, CD4, and CD8 immunocytes in the tumor tissues of the rats exposed to hyperthermia. Heat shock protein 70 also appeared in the viable area at its boundary with the necrotic area. Further study showed magnetic liposomes plus alternating magnetic field heat therapy suppressed tumor growth in bone microenvironment; however, almost half of the animals that received magnetic liposomes plus alternating magnetic field treatment died. This method has some side effects and needs further study. Gold nanoshells are designed to absorb near-infrared light that strongly generates heat and provides optically guided hyperthermic ablation. Laser-activated gold nanoshells have been shown to kill human prostate cancer cell PC-3 and C4-2 in vitro, and in vivo study with ectopic murine prostate cancer model with laser-activated gold nanoshells caused 93% necrosis and regression in the high dose of gold nanoshell–treated mice.
Several clinical trials of nanocarrier-based delivery of chemotherapeutic drugs are underway. One multi-institutional phase II trial of pegylated-liposome doxorubicin in the treatment of locally advanced unresectable or metastatic transitional cell carcinoma of the urothelial tract showed clinical response rate and favorable toxicity profile. Liposomal doxorubicin was used to treat hormone-refractory prostate cancer, which is a challenge to urologists. The phase I study in which liposome-encapsulated doxorubicin was used to treat patients with advanced, androgen-independent prostate cancer did not show clinical response, but that may be because of the low dosage. In another prospective randomized phase II trial, however, patients with symptomatic hormone-refractory prostate cancers were treated with pegylated liposomal doxorubicin at 25 mg/m 2 every 2 weeks for 12 cycles (group A) or 50 mg/m 2 every 4 weeks for 6 cycles (group B). Decrease of prostate-surface antigen level was observed in 25.8% patients in group B, the mean time to disease progression was 6.5 months, patients in group B had a significantly higher rate of pain relief, and the mean 1-year survival rate was also significantly higher. Toxicity types differed significantly between groups A and B, but no dose-limiting cardiotoxicities or hematotoxicities were found.
Nanocarrier Delivery of Drug for Noncancer Diseases
Nanocarrier delivery of drug not only can be used to treat cancer but also benign diseases. The effect of liposomes prepared from various natural and synthetic lipids on attenuating hyperactivity in bladder irritation was studied. Liposome of uncharged zwitterionic phospholipids significantly attenuated the irritation and decreased bladder contrast frequency caused by protamine sulfate but empty liposomes were not able to achieve the same effect.
Effect of intraurethral application of prostaglandin E 1 –loaded liposome was compared with that of intracavernosal injection of prostaglandin E 1 for treating psychogenic and organic erectile dysfunction. The intraurethral application of liposomal prostaglandin E 1 was not effective in patients with organic erectile dysfunction. In 60% of patients with psychogenic erectile dysfunction, however, it was effective enough. It might be a convenient and painless therapeutic alternative for selective groups of patients. Foldvari and colleagues tested the effect of transdermal delivery of liposome-encapsulated prostaglandin E 1 in patients with erectile dysfunction. The study was performed in five patients in a double-blind, placebo-controlled fashion. Application of two transdermal prostaglandin E 1 formulations caused peak systolic flow velocities in the deep cavernosal arteries of patients to increase significantly. The highest mean peak systolic flow velocity was achieved at 45 minutes after application. Formulation of liposome with encapsulated prostaglandin E 1 showed sevenfold increase mean peak flow velocity compared with baseline values following transdermal application of the formulation, and could be a promising approach for the treatment of erectile dysfunction.
Significantly improved survival of rats with renal transplantation was shown following intravenous administration of bilayer liposome-encapsulated methylprednisolone once a week. Daily administration of the same dose of free drug could result in similar survival, but urine analysis showed a consistently higher protein excretion and retention of creatinine and urea in the free-drug group. Compared with free methylprednisolone, liposome-encapsulated methylprednisolone may selectively inhibit T-cell activation and cytokine production. The expression of CD45RC, CD25, interleukins-2, -7, and -12, tumor necrosis factor-α, and interferon-γ was strongly inhibited in the drug-encapsulated group.
Nanocarrier for Delivery of Genes
Gene therapy refers to the transfer and expression of genes of therapeutic applications in the target cells and is regarded as a potential revolution in medicine. The application of gene therapy promises progress in understanding physiologic roles of genes and in treating diseases at the genetic level. The ectopic expression of foreign genes is the most critical aspect for the success of in vivo gene expression and therapy. The vectors, which protect the ectopic genetic material and ferry it to the cells, are classified into two categories: viral and nonviral. Viral vectors have efficient cell-entry mechanism; however, these viral vectors have several major restrictions, such as limited DNA-carrying capacity, lack of target-cell specificity, immunogenicity, and the risk of insertional mutagenesis. Nonviral vectors are relatively simple to synthesize and have fewer risks than their viral counterparts. In addition, the nonviral vectors have no limitation on the size and number of the genetic inserts. Nanocarriers are promising used as nonviral vectors.
Prabha and Labhasetwar studied the parameters that influence the efficacy of nanoparticle-mediated gene transfection. The results showed the DNA loading in nanoparticle and its release, and surface properties of nanoparticles are the critical determinant in nanoparticle-mediated gene transfection. Larchian and colleagues developed a liposome-mediated immune gene therapy using interleukin-2 and B7.1 in a MBT-2 mouse bladder cancer model. The study showed that the liposome-mediated transfection is safe, simple, and highly effective compared with retroviral system. Herpes simplex virus thymidine kinase (HSV-tk) gene delivered by folate-linked, lipid-based nanoparticles can achieve high transfection efficiency and selectivity, inhibiting tumor growth following intratumoral injection into prostate cancer.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree






