Continuous innovations and clinical research in ultrasound (US) technology have upgraded the position of US in the imaging armamentarium of urologists. In particular, contrast-enhanced US and sonoelastography seem to be promising in the diagnosis of urologic cancers, implementation of ablative treatments, and monitoring of treatment response. This article focuses on the potential clinical applications of recent advances in US technology in oncologic urology.
Ultrasonography (US) represents a key instrument in the diagnostic armamentarium of urologists because it is rapid, effective, noninvasive, radiation-free, and relatively inexpensive. During the past 15 years, continuous innovations in transducer engineering, electronics, computers, signal processing, and the development of contrast agents have resulted in advances in image resolution, three-dimensional (3D) and four-dimensional US, harmonic imaging, compound imaging, contrast-enhanced (CE) US, and sonoelastography. In light of these developments, there is an increasing flow of data from studies investigating the role of US in the diagnosis, management, and follow-up of urologic patients. This article indicates the recent advances in US technology and focuses on their potential clinical applications in oncologic urology.
Advances in the diagnosis of prostate cancer
Three-Dimensional Imaging
3D technology offers several advantages over two-dimensional (2D) transrectal ultrasound (TRUS) imaging of the prostate, such as improving diagnosis of prostate cancer (PCa), assessment of brachytherapy seed placement or treatment mapping, cryoablation guidance, accurate diagnosis of extraprostatic tumor extension, and staging.
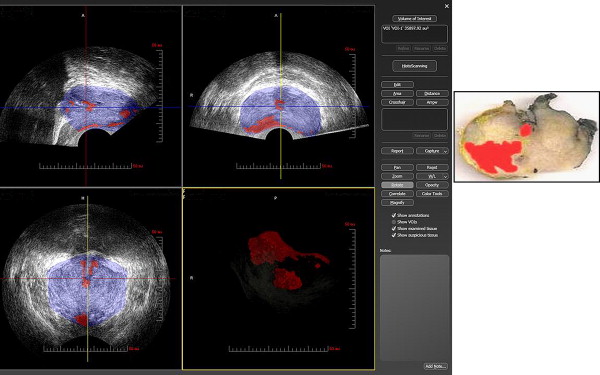
Prostate HistoScanning is a novel computer-aided ultrasonographic technology based on tissue characterization algorithms. The characterization algorithms exploit the physical changes to sound waves that result from the interaction of the US beam and the cancer tissue (energy loss, erratic spatial energy distribution, and increased entropy). These can be applied in discrete regions of interest in the prostate. Thus, the presence of PCa can be ascertained within minute discrete tissue volumes. Prostate HistoScanning enables determination of PCa location and volume ( Fig. 1 ). It has been reported that foci 0.50 mL or greater can accurately be detected, and this technology has been proposed as a potential triage test for men deemed to be at risk for PCa who wish to avoid biopsy.
Elasticity Imaging
The use of US-based elasticity imaging for the detection of PCa relies on the fact that these tumors can be detected because they are firmer than the surrounding normal parenchyma ( Fig. 2 A ). The results of several recent studies are promising. These studies can be schematically divided into two conceptually overlapping categories. The first category is studies based on images (no targeted biopsies obtained) to evaluate the technique as a diagnostic tool for PCa detection. Most of these studies showed that elastography improved the detection rate of PCa, and the detection increased with higher Gleason scores. Detailed results are displayed in Table 1 . The second category is studies based on elastography-targeted biopsy results to evaluate the method as a tool to support or replace TRUS-guided systematic random biopsy.
| Reference | Patients (PCa) | Reference Standard | E Compared with | Main Study Results |
|---|---|---|---|---|
| Taylor et al, 2005 a | 19 | RPS | 3D-US | 3D-E has a better diagnostic performance for PCa tumors ≥1 cm 3 |
| Miyanaga et al, 2006 | 29 b | Biopsy | TRUS/DRE | Detection rates: E (93%), c TRUS 55%, DRE (59%) |
| Two patients missed had well-differentiated PCa | ||||
| 11 d | Detection rates: E (55%), TRUS (55%), DRE (64%) in previously treated patients | |||
| Pallwein et al, 2007 | 15 | RPS | — | 92% diagnostic accuracy, 80% overall sensitivity (100% at apex) |
| Tsutsumi et al, 2007 | 51 | RPS | TRUS | Detection rates: E (84%), c TRUS (31%); detection rate increases to 100% if modalities are combined |
| Anterior tumors: excellent detection; for higher grade tumors, the detection rate is lower | ||||
| Sumura et al, 2007 | 17 | RPS | TRUS/DRE | Detection rates: E (74.1%), c TRUS (48.1%), DRE (33.3%), CD (55.6%), MRI (47.4%) |
| CD/MRI | No difference in detection rates for anterior-posterior sides but higher for higher Gleason scores or tumor volumes | |||
| Pallwein et al, 2008 | 492 | Biopsy | — | 87% sensitivity, 72% specificity (best at apex); good correlation with systematic biopsy results |
| Salomon et al, 2008 | 109 | RPS | — | 75% sensitivity, 77% specificity, 76% accuracy; E findings correlate best at apex |
| Higher detection rates for higher Gleason score (up to 93% for scores >7) | ||||
| Eggert et al, 2008 e | 351 | Biopsy | TRUS | E predicted histopathologic findings in only 44.5% of the cases; E does not improve detection rate |
b Previously untreated patients.
c Elastography superior to other modalities.
d Patients treated previously with hormone therapy. Elastography detection rate dropped, possibly attributable to softer rendered consistency of lesion by treatment.
e Randomized clinical trial in which both arms have been submitted to conventional TRUS-guided 10-core biopsy. One arm has additionally offered elastography before biopsy.
König and colleagues evaluated 404 men with elastography in addition to a conventional TRUS-guided systematic sextant biopsy protocol. It was concluded that the sensitivity using this technique in conjunction with conventional diagnostic methods for guided biopsies is high (84.1% versus 64.2%). Elastography-targeted prostate biopsy was directly compared with systematic biopsy guided by conventional gray-scale TRUS in 230 screening volunteers. The detection rate of patients who had PCa did not differ significantly between targeted and systematic biopsies (30% versus 25%, respectively). Detection rates per core differed significantly (targeted versus systematic: 12.7% versus 5.6%), however. A patient who has PCa was found to have a 2.9-fold higher probability of being detected by targeted compared with systematic biopsy.
To compare the detection rate of PCa and the distribution of Gleason scores, targeted biopsies based on conventional TRUS, color Doppler (CD) US, and elastography were obtained, along with systematic sextant biopsies, in 137 patients. The detection rate was higher for targeted than systematic cores, and positive results on CD-US and elastography were strongly associated with high-grade and moderate- to high-grade cancers, respectively. It was concluded that although CD-US and elastography may enhance PCa detection, a targeted biopsy protocol alone is not sufficient to replace the conventional sextant biopsy protocol.
Kamoi and colleagues compared the performance of elastography with conventional TRUS and power Doppler (PD) US in PCa detection by evaluating a clinical population of 107 men. TRUS sensitivity was significantly lower (50%) than that of PD-US (70%) or elastography (68%), whereas the accuracy was comparable among these modalities, ranging from 72% (TRUS) to 76% (elastography). Core analysis revealed a significantly lower detection rate for systematic biopsies compared with the combination of PD-US-elastography, however. It was concluded that elastography may complement conventional US to minimize the number of cancers missed.
In conclusion, based on the available results, it seems that transrectal elasticity imaging allows for targeted prostate biopsies and may reduce the number of needed cores to detect cancer. Nevertheless, further clinical trials are still necessary to define the advantages and the exact role of this relatively novel technique better in the diagnosis of PCa.
Contrast-Enhanced Ultrasonography
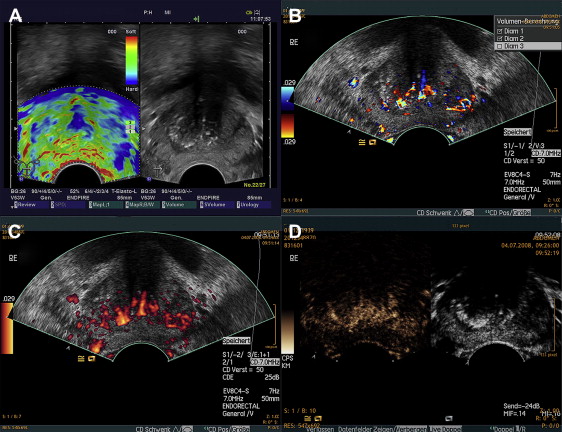
Microbubble-based contrast agents visualized with novel “contrast-specific” imaging modes represent a revolution in ultrasound. Contrast agents may facilitate the detection of PCa ( Fig. 3 ), providing additional information on tumor size or aggressiveness, in addition to the monitoring of antiangiogenic treatment results, because of the increased vascularization of PCa, which can be visualized with CE-US. An example of the depiction of a prostate tumor with the use CD-US, PD-US, and cadence contrast-pulse sequence (CPS) in the same patient is presented in Fig. 2 B through D, respectively.
To determine the role of CE-US in PCa detection, localization, and treatment follow-up, a multicenter research coordination project has been conducted in four European countries during the period 2002 through 2006 (CONTRAST, QLRT-2001-2174). Recently, the results based on 3746 patients have been reported in comparison to published data outside this research group. The individual studies of the project used various contrast agents imaged by CD-US, 3D-PD-US, and novel nonlinear imaging techniques (CPS).
The correlation of histologic findings in radical prostatectomy specimens with preoperative CE-US images regarding tumor localization is promising. It seems that technical improvements have increased sensitivity for the detection of perfusion patterns. Lesions with increased microvascular density may be visualized with 3D-CE-PD-US and CE-CD-US. It has been reported that 3D-CE-PD-US is a superior diagnostic tool compared with digital rectal examination, prostate-specific antigen (PSA), and gray-scale US or PD-US and that the most suitable diagnostic predictor for PCa is the combination of 3D-CE-PD-US and PSA. Despite improving sensitivity for PCa detection, CE-US may also demonstrate focal enhancement in areas of benign hyperplasia. Nevertheless, accurate detection of localized tumors with 3D-CE-PD-US has been reported in up to 78% of patients. In a study including 70 patients evaluated with the same technique, diagnosis by imaging alone has been improved from 61% with standard investigations to an average of 86% of tumors, with detection of foci 5 mm or greater in 68% to 79%. In another small series, all T3 tumors could be identified using microvascular imaging.
The clinical value of these initial results should be tested in a diagnostic setting. The effect of CE-US on the prostate biopsy protocols regarding the detection rate has been extensively investigated. The combined European research project demonstrated a clear association between prostate contrast enhancement and diagnosis of clinically significant PCa. The sensitivity is increased with the use of CE-US targeted biopsies; fewer biopsies are needed to obtain the same detection rate, and the tumors detected have a higher Gleason score than those detected by random biopsies.
These findings are in agreement with the results from studies outside the European research project. Using various CE-US methods, including novel nonlinear imaging techniques (intermittent harmonic imaging, flash replenishment, and CPS), these studies directly compared gray-scale TRUS-guided systems with CE-US–targeted biopsy protocols ( Table 2 ) or investigated the contribution of CE-US on the systematic biopsy protocols and its value in predicting the nature of hypoechoic lesions.
| Reference | Patient Analysis | Biopsy Core Analysis | CE-US Imaging Technique | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Total | PCa (%) | TB Versus SB | Total | TB | PCa (%) | TB Versus SB | ||||||||
| TB | SB | TB + SB | P Value | Total | TB | SB | OR (95% CI) | P Value | ||||||
| European research coordination project | Frauscher et al, 2002 | 230 | 24.4 | 22.6 | 30.0 | ns | 3439 | 1139 | 7.0 | 10.4 | 5.3 | 2.6 (1.9–3.5) | <0.001 | CD |
| Pelzer et al, 2005 | 380 | 27.4 | 27.6 | 37.6 | ns | 5700 | 1900 | 8.6 | 32.6 a | 17.9 a | 3.1 (na) | <0.01 | CD | |
| Mitterberger et al, 2007 b | 690 | 26.1 | 24.1 | 32.0 | ns | 10,317 | 3417 | 7.6 | 11.1 | 5.8 | na | <0.001 | CD | |
| Mitterberger et al, 2007 c | 100 | 32.0 | 26.0 | 29.0 | 0.04 | 750 | 250 | 9.7 | 15.6 | 6.8 | — | <0.001 | CD | |
| Mitterberger et al, 2008 d | 36 | 33.3 | 16.7 | 33.3 | 0.04 | 540 | 180 | 12.2 | 17.0 | 10.0 | 2 (na) | 0.027 | CD | |
| Pallwein et al, 2008 | 20 | 40.0 | 25.0 | 40.0 | ns | na | na | na | na | na | na | na | CPS | |
| Frauscher et al, 2001 | 84 | 27.4 | 20.2 | 28.6 | 0.034 | 1249 | 409 | 7.7 | 13.4 | 4.9 | 4.3 (2.6–7.1) | <0.001 | CD | |
| Halpern et al, 2005 e | 301 | 27.6 | 30.9 | 34.6 | ns | 2939 | 1133 | 363 | 15.4 | 10.4 | 2.0 (na) | <0.001 | CD/PD/CHI/IHI | |
| Linden et al, 2007 | 60 | 21.7 | 26.7 | 30 | ns | 825 | 225 | 9.6 | 12.9 | 8.3 | 2.0 (na) | 0.034 | MFI | |
| Colleselli et al, 2007 | 345 | 77.4 | 73.0 | na | ns f | 5175 | 1725 | — | — | — | — | — | CD | |
a Detection rate based on number of positive cores per total number of cores in the 143 men with PCa detected by the combined approach.
b Impact on Gleason score was studied: TB detected significantly higher Gleason scores compared with SB and may allow for identification of more aggressive tumors.
c Only randomized clinical trial to date. Two arms of 50 men underwent systematic or CE-targeted biopsies of the prostate.
d Short-term (14 days) application of dutasteride may be promising to improve cancer detection by CE- targeted US-guided biopsies because of blood flow reduction in benign prostatic tissue.
e IHI provides a statistically significant advantage over gray-scale and Doppler imaging but not over CHI. It significantly improves the characterization of tissue as benign versus malignant and may therefore improve PCa detection, but the technique is not sufficient to differentiate benign from malignant tissue definitely without biopsy confirmation.
f Statistically significant difference in PCa detection rate in favor of CE-US was detected only in small prostate glands (69% versus 88.1% and 70.4% versus 80.8% for prostate volumes of <20 mL and 20–30 mL, respectively).
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree






