The use of bioadhesives, tissue sealants, and hemostatic agents has allowed for increased use of minimally invasive techniques for complex reconstructive urologic procedures. Hemostatic agents can facilitate clot formation through enzymatic reactions with host factors, mechanical compression, or a combination of the two. Tissue sealants and bioadhesives act through polymerization between themselves and adjacent tissues. This article reviews the unique features, mechanism of action, safety profile, and prototypical applications of the agents most commonly used in urologic surgery.
This article is not certified for AMA PRA Category 1 Credit ™ because product brand names are included in the educational content. The Accreditation Council for Continuing Medical Education requires the use of generic names and or drug/product classes as the required nomenclature for therapeutic options in continuing medical education.
For more information, please go to www.accme.org and review the Standards of Commercial Support.
The past two decades have witnessed a substantial growth in the use of bioadhesives, tissue sealants, and hemostatic agents across all disciplines of surgical practice. This growth has been encouraged by two major trends in surgical practice: an expansion of minimally invasive surgery and increasingly complex reconstructive procedures. Minimally invasive surgical techniques are more limited than open surgical ones in their capacity to obtain hemostasis, and hemostatic agents can help make up the deficit. Similarly, the outcomes of complex reconstructive procedures can be enhanced by bioadhesives and tissue sealants. The greatest applications of these agents, however, are when these two trends are combined, for minimally invasive complex reconstructive surgery. These procedures have been made possible in large part through the development of novel materials that help control blood loss and promote tissue healing.
Typically, the use of a specific bioadhesive, tissue sealant, or hemostatic agent has been based on the personal experience and training of individual physicians, and in many cases these applications are off-label. Despite their widespread use, there remains significant confusion regarding the appropriate indications for the use of individual agents. This article discusses the unique features, mechanism of action, safety profile, and several applications of the agents most commonly used in urologic practice. This article is not intended to be an exhaustive summary of all reported urologic uses of bioadhesives, tissue sealants, and hemostatic agents. Instead, it describes the evidence for a set of prototypical applications to guide the reader in the appropriate selection of a particular product based on a specific clinical situation.
Enzymatic hemostasis
Hemostasis and Thrombosis
Thrombus formation and hemostasis in the setting of vascular injury requires two separate components : plasma clotting factors to generate fibrin and platelets to form a hemostatic plug. The coagulation cascade consists of a tightly regulated set of enzymes, which results in the cleavage of prothrombin into its active form, thrombin. Thrombin ultimately causes enzymatic cleavage of soluble plasma fibrinogen into fibrin monomers. The fibrin then forms cross-linked polymers through enzymatic interactions with factor XIII. This cross-linked polymer is insoluble and forms a framework to which platelets can activate and adhere, forming a hemostatic plug. This process is shown schematically in Fig. 1 . There are several conditions, both acquired and inherited, which cause the coagulation cascade to be deficient. Examples of acquired conditions include hypothermia, consumptive coagulopathy, and pharmacologic anticoagulation. Inherited conditions include both qualitative (von Willebrand disease) and quantitative defects (hemophilia) in coagulation factors.
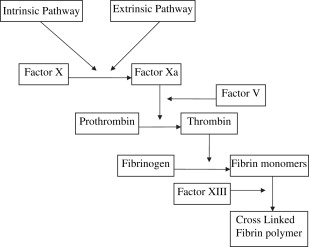
The goal of all enzymatic hemostatic agents is to mimic the final step of the coagulation cascade in which fibrinogen is cleaved by thrombin to form a fibrin clot. This bypasses the complicated sequential activation of multiple coagulation factors and allows for the delivery of active end products to the site of action allowing for efficient, localized coagulation. Fibrinogen or thrombin are ideally delivered to the site of injury by topical administration for two reasons: first, direct application to the site of injury allows for supraphysiologic concentration of specific prothrombotic agents producing the largest therapeutic effect in controlling hemorrhage; second, local application reduces the risk of unintended prothrombotic complications potentially seen with systemic administration of prothrombotic agents.
Fibrin Sealants
All commercially available fibrin sealant preparations consist of two major components: thrombin and fibrinogen. The supraphysiologic concentration of fibrinogen in these preparations (15–25 times higher than in the circulating plasma) allows for rapid and predictable clot formation. The two components are then delivered using a dual-chambered delivery system allowing each to be applied simultaneously. Specific preparations may also include antifibrinolytics, which act to preserve the clot against enzymatic degradation.
Currently, there are two commercially available fibrin sealant products in the United States: Tisseel (Baxter Health Care, Deerfield, Illinois) and Evicel (Johnson and Johnson, West Somerville, New Jersey). Both products include human donor fibrinogen and thrombin, but they differ in their mechanism of antifibrinolysis. Tisseel contains the antifibrinolytic aprotinin. This was originally derived from bovine lung, but current formulations contain a completely synthetic aprotinin because of concerns regarding allergic reactions to bovine proteins. Aprotinin is a serine protease inhibitor that limits fibrinolysis through inhibition of plasmin, kallilrein, and trypsin. Evicel does not use any specific antifibrinolytic; rather, plasminogen (the enzyme responsible for enzymatic cleavage of fibrin polymers) is removed through chromatographic filtering eliminating the need for an additional antifibrinolytic. All fibrin sealants are contraindicated for intravascular use, because the combination of fibrinogen and thrombin can induce systemic thrombosis. Fibrin sealants work best when applied to a dry (bloodless) surgical field, which is in contrast to thrombin-based hemostatic agents (see later).
The major safety concern regarding commercially available allogenic fibrin sealants relates to their use of donor plasma for obtaining fibrinogen and thrombin, with the associated risk of viral transmission. Following screening of donor plasma for hepatitis B and C and HIV, all available human-derived products undergo treatment to inactivate potential viral particles. In the case of Tisseel, this includes two-step vapor heating at 60°C and 80°C for both fibrinogen and thrombin. For Evicel, this includes solvent detergent treatment followed by pasteurization (fibrinogen) or nanofiltration (thrombin). There have been no documented cases of viral transmission from fibrin sealants from Food and Drug Administration (FDA)–approved agents, so it seems that the viral inactivation procedures are effective.
Nonetheless, in response to the concerns over infectious disease transmission, products have been developed that use autologous blood to derive thrombin and fibrinogen. Currently, there are two FDA-approved methods for obtaining autologous fibrin sealant. The first is Vitagel (Orthovita, Malvern, Pennsylvania), which is composed of microfibrillar bovine collagen and thrombin. This is combined with approximately 10 mL of the patient’s own plasma as a source of fibrinogen, which can be prepared in approximately 5 to 7 minutes intraoperatively. The resulting collagen and fibrin scaffold is then absorbed in approximately 30 days. Although this product does not use any allogenic human components, it does use bovine collagen and thrombin, and it should not be used in patients with allergic reactions to bovine products.
The other method for generating autologous fibrin sealant uses blood bank devices that concentrate thrombin and fibrinogen from a patient’s own plasma. This system, marketed as CryoSeal (Thermogenesis, Rancho Cordova, California), requires 60 minutes to produce fibrin sealant, but the sealant can be stored frozen at −18°C for up to 1 year. The CryoSeal system uses approximately 400 mL of autologous plasma, whereas the VivoStat system (VivoStat A/S, Denmark, not currently available in the United States) uses similar technology but requires only 120 mL of plasma and requires approximately 30 minutes to prepare. Although the fibrin and thrombin concentration is significantly lower in the clots formed from CryoSeal and VivoStat systems compared with Tisseel, maximum clot strength was similar between CryoSeal-derived fibrin sealant and Tisseel. A limitation of the CryoSeal system is that because it uses a patient’s own coagulation factors, it is contraindicated in patients with acquired or inherited coagulation disorders, or a recent history of anticoagulation.
The first reports of the use of fibrin sealants for hemostasis in renal parenchymal injuries was described in the setting of renal trauma by Kram and colleagues. In their series of 14 patients with penetrating renal trauma, they noted that fibrin glue was able to prevent hemorrhage and urinary leak in all patients with no complicating infections, urinary fistulae, rebleeding episodes, or urinary obstruction. Fibrin sealants can also be useful in iatrogenic trauma. Canby-Hagino and colleagues described two patients who suffered splenic injuries during laparoscopic partial nephrectomy (LPN) treated with fibrin sealant. In both cases, bleeding was able to be controlled and splenectomy was avoided. Several reports have documented the efficacy of fibrin sealants in obtaining hemostasis during partial nephrectomy. Levinson and colleagues reported no hematoma or abscess formation in seven patients undergoing open partial nephrectomy. The use of hemostatic agents during LPN becomes even more important because achieving hemostasis though the use of direct pressure can be difficult or impossible (except for hand-assisted techniques). Pruthi and colleagues found that in 15 patients undergoing LPN, application of fibrin sealant following argon beam electrocautery was successful in obtaining hemostasis in all cases. Johnston and colleagues found that when the collecting system or renal sinus was entered during LPN, fibrin sealant (plus a gelatin sponge, see later) resulted in significantly higher rates of hemorrhage or urine leak compared with sutured bolster. They concluded that although fibrin glue alone is acceptable for closure of superficial LPN parenchymal defects, sutured bolster is recommended when there is collecting system or renal sinus entry. Fig. 2 shows fibrin glue applied to a porcine LPN defect. Schips and colleagues have reported the successful use of autologous fibrin sealant for hemostasis in 10 patients undergoing LPN using the VivoStat system. Although the results of these studies are difficult to compare because of the lack of adequate control groups and the use of additional hemostatic methods in addition to fibrin glue (eg, argon beam coagulator, bipolar cautery, and mechanical hemostatic materials), the results do suggest that fibrin sealant is useful in obtaining hemostasis in the setting of superficial LPN, and as an adjunct in closure with sutured bolster when the collecting system or renal sinus is entered.
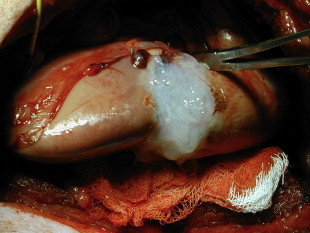
In addition to its role in hemostasis, fibrin sealants have the ability to induce fibroblast cellular migration and growth factor induction making them ideal for use as a urinary tract sealant where a water-tight seal is required.
Initial animal experiments demonstrated that the use of fibrin sealant to close a pyeloplasty was superior to laser tissue welding in terms of leak pressure, histologic appearance, flow characteristics, and operative time. Subsequently, Eden and colleagues performed the first series of laparoscopic pyeloplasty using fibrin glue in eight patients. In this study, sutures were placed to approximate the two edges and fibrin glue was used to complete the anastomosis. The investigators found that operative time was decreased compared with historic controls (142 versus 193 minutes) and all anastomoses were patent on diuretic renography at 1 to 2 years follow-up.
Fibrin sealant has been used in both simple and radical retropubic prostatectomy. In simple prostatectomy, Morey and colleagues applied fibrin sealant to the prostatic capsule of five patients allowing for a drain-free closure without apparent adverse effects. Diner and colleagues showed that fibrin sealant applied following radical retropubic prostatectomy significantly decreased the drain output compared with controls ( P <.001). The small sample size in these cohorts and recent evidence that routine pelvic drainage may not be necessary at all suggest, however, that these studies were underpowered to detect small differences between the groups. Further evidence from large, randomized trials is required before it can be concluded that fibrin sealant provides appreciable reduction of anastomotic leakage in the setting of prostatectomy.
The sealant properties of fibrin glue can also be used to promote chronic wound healing by increasing tissue adherence, reducing dead space, inducing fibroblast migration and differentiation, and accelerating revascularization. The first application of fibrin sealant to close a urinary fistula was in 1985 by Papadopoulos and colleagues in an artificial vesicovaginal fistula in a rabbit model. In their study, sutured closure was associated with a 30% recurrence rate, whereas no recurrences were noted in the group closed with fibrin sealant. Since then there have been numerous small case reports of attempts at closure of urinary fistula using fibrin sealants, generally with favorable results even in the setting of prior pelvic irradiation. Evans and colleagues reported their use of fibrin sealant in six patients (five males) with complex urinary fistulas. All patients in this group had successful repair of their fistula with a combination of fibrin sealant and surgical correction. In addition to closure of lower urinary tract fistulae, fibrin sealant has been used successfully to close a persistent nephrocutaneous fistula following partial nephrectomy.
Thrombin
Thrombin acts through the enzymatic cleavage of fibrinogen to fibrin creating an insoluble clot to which platelets can adhere. The major difference between the commercially available thrombin preparations and fibrin sealants is the absence of fibrinogen in the former. Thrombin is most efficacious when there is blood present in the field to provide the necessary fibrinogen for enzymatic hemostasis. Three sources of thrombin have been used: (1) bovine, (2) human pooled plasma, and (3) recombinant. Table 1 provides a list of commercially available thrombin agents. The efficacy of the various thrombin preparations has been compared in several randomized trials, although none was placebo controlled. No significant difference was noted between human plasma and bovine-derived thrombin in a phase III, double-blinded trial with greater than 90% of the subjects in each arm achieving hemostasis within 6 minutes. Recombinant thrombin has been similarly compared with bovine-derived thrombin. Again, this trial showed no difference in time to hemostasis between the recombinant group and the bovine group (95.4% versus 95.1% hemostasis within 10 minutes, respectively).
| Class | Brand Name | Manufacturer |
|---|---|---|
| Enzymatic agents | ||
| Fibrin | Tissee | Baxter Health Care |
| Evicel | Johnson & Johnson | |
| Vitagel | Orthovita | |
| CryoSeal | Thermogenesis Corporation | |
| Thrombin | ||
| Bovine | Thrombin-JMI | King Pharmaceuticals |
| Human | Evithrom | Johnson & Johnson |
| Recombinant | Recothrom | Zymogenetics |
| Gelatin matrix | FloSeal | Baxter Health Care |
| Cross-linking sealants | ||
| Polyethylene glycol | CoSeal | Baxter Health Care |
| Albumin-gluteraldehyde | BioGlue | CryoLife |
| Cyanoacrylate | Dermabond | Johnson & Johnson |
| Mechanical Scaffold | ||
| Porcine gelatin | Gelfoam | Pfizer |
| Surgifoam/Surgifl | Johnson & Johnson | |
| Collagen | Avitine | Davol |
| Helistat/Helitene | Integra | |
| Instat/Ultrafoam | Johnson & Johnson | |
| Oxidized cellulose | Surgicel | Johnson & Johnson |
| Nu-Knit | ||
| Fibrillar | ||
| Original | ||
The safety concerns relating to the use of allogenic human blood products have been previously discussed. Another safety concern relates to the use of bovine-derived products. These products can induce allergic reactions, especially when used repeatedly in a single patient. In patients with known hypersensitivity to bovine products the use of products containing bovine proteins is contraindicated. Additionally, the antibodies formed following exposure to bovine thrombin (or factor V contaminants found in small amounts in bovine-derived thrombin preparations) can cross-react with human clotting factors and induce severe coagulopathy. Recombinant thrombin has a significantly lower incidence of development of specific antiproduct antibodies compared with bovine thrombin (1.5% versus 18%, respectively). Additionally, patients who formed antibovine thrombin antibodies were shown to have increased bleeding, thrombotic events, and abnormal activated partial thromboplastin time values compared with those who formed antirecombinant thrombin antibodies. The recent trend to replace bovine products with recombinant or human-derived proteins avoids this complication.
Although thrombin alone has been shown to be effective in hemostasis when blood is present in the surgical field (Thrombin-JMI PI), most studies have used it in combination with an absorbable gelatin matrix. FloSeal (Baxter Health Care, Deerfield, Illinois) uses a combination of human thrombin and bovine gelatin to provide a scaffold with both enzymatic and mechanical hemostatic properties. The gelatin matrix granules swell by as much as 20% within 10 minutes when placed in contact with blood allowing for conformation to the shape of a wound, providing both mechanical tamponade and a scaffold for the resulting fibrin polymer. Fig. 3 shows FloSeal applied to a partial nephrectomy defect in a porcine model. The matrix is biocompatible and is resorbed within 6 to 8 weeks. The question still remains, however, as to whether the enzymatic or mechanical properties provide most of the hemostatic function. Although there has not been a randomized, controlled trial comparing thrombin plus gelatin sponge, thrombin plus gelatin matrix, and gelatin matrix alone, comparison of individual trials shows roughly equivalent efficacy in terms of hemostasis at 10 minutes (97%, 96%, and 97%, respectively).
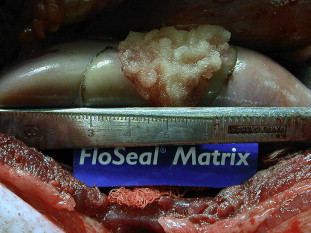
The urologic applications of gelatin matrix thrombin agents (FloSeal) have been most extensively studied in LPN. In a porcine model, Desai and colleagues found that application of gelatin matrix following LPN resulted in complete hemostasis without the need for renal ischemia despite significant collecting system entry. Gill and colleagues compared outcomes of patients who had FloSeal applied to the surgical defect before performing sutured renorrhaphy with those in which FloSeal was not used. They found that FloSeal application was associated with a decreased risk of overall complications (37% versus 16%; P = .008) and a trend toward fewer hemorrhagic events (12% versus 3%; P = .08).
The use of other topical thrombin-based hemostatic agents has not been well described in the urology literature. Typically, stand-alone topical thrombin agents are used in conjunction with an absorbable gelatin sponge to provide a scaffold. The reason for the popularity of FloSeal over other thrombin preparations with absorbable gelatin sponges may be the ease of application of a flowable preparation to an irregular surgical defect.
Although surgical exploration for high-grade renal trauma has classically resulted in nephrectomy, the availability of hemostatic agents has sparked interest in renal preservation for these injuries. Hick and colleagues induced a complex upper pole renal injury in a porcine model and compared FloSeal with conventional sutured repair. They found that the use of FloSeal was associated with significantly reduced overall blood loss and time to hemostasis with no delayed bleeding or urine leak. Although these laboratory results are promising, the role of hemostatic agents for renal salvage therapy has not been investigated in human subjects.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree






