Recently, advances in imaging technology have made possible the ability to image noninvasively specific molecular pathways in vivo that are involved in disease processes. Molecular imaging evaluates changes in cellular physiology and function rather than anatomy, which are likely to be earlier and more sensitive manifestations of disease. In addition, as newer drugs to treat disease become increasingly molecule specific, molecular imaging has become necessary to provide noninvasive determination of patients likely to benefit from treatment and early therapy response. This article reviews current and emerging molecular imaging technologies relevant to urologic surgery.
Imaging plays an important role in the field of urologic surgery and is routinely used to diagnose genitourinary disease, stage urologic malignancies, and guide interventions. Multiple imaging modalities are currently used, including conventional radiography, x-ray fluoroscopy, ultrasound, CT, and MRI. These imaging technologies all image structural anatomy by exposing the body to energy and resolving intrinsic contrast differences among tissues. Recently, advances in imaging technology have made possible the ability to image noninvasively specific molecular pathways in vivo that are involved in disease processes. Molecular imaging evaluates changes in cellular physiology and function rather than anatomy, which are likely to be earlier and more sensitive manifestations of disease. In addition, as newer drugs to treat disease become increasingly molecule specific, molecular imaging has become necessary to provide noninvasive determination of patients likely to benefit from treatment and early therapy response. This article reviews current and emerging molecular imaging technologies relevant to urologic surgery.
Molecular imaging methods
Positron Emission Tomography
Positron emission tomography (PET) uses compounds labeled with positron-emitting radioisotopes as molecular probes. The most commonly used clinical PET probe is the 18 F-labeled glucose analogue 2- 18 F-2-deoxy-D-glucose (FDG). This probe is internalized into cells through glucose transporters and phosphorylated; however, the phosphorylated product does not undergo further processing and is retained within cells in proportion to their rate of glycolysis. Many tumor cells are known to have increased glycolytic rates relative to normal cells even under aerobic conditions (known as the Warburg effect), which is thought to confer a selective advantage to transformed cells by allowing higher rates of proliferation and facilitating invasion and metastasis. This association of increased glycolysis with malignancy forms the rationale for using FDG-PET as a method of detecting tumors. FDG-PET imaging of some urologic malignancies has been problematic. For example, FDG-PET has been shown to have lower sensitivity for prostate cancer detection compared with CT. Additional studies have failed to demonstrate high accuracy rates of FDG-PET for distinguishing prostate cancer from benign prostate hypertrophy (BHP) or for detecting prostate cancer recurrence after surgery. The relative indolence of prostate cancer, associated with low levels of tumor cell glycolysis, has been used to explain the poor detection rates with FDG-PET. PET imaging with 11 C-choline, which accumulates in proliferating tumor cells undergoing increased cell membrane lipid synthesis, may prove to be superior to FDG-PET for detecting primary prostate malignancies and metastases. FDG-PET imaging of renal cell carcinoma (RCC) and transitional cell carcinoma is limited by the renal excretion of tracer, which obscures detection of urinary tract tumors. PET imaging with other metabolic tracers that do not undergo urinary excretion, such as 11 C-methionine, are being explored for detection of urinary tract malignancies.
Lymphotropic Nanoparticle-Enhanced MRI
Lymphotropic superparamagnetic iron oxide particles (USPIO) consist of a monocrystalline superparamagnetic iron oxide core coated with dextrans to prolong circulation time. These particles, when injected intravenously, are taken up by lymphatic vessels and accumulate within lymph nodes, wherein they are internalized by macrophages. Normal uptake of USPIO in lymph nodes results in magnetic susceptibility effects of iron oxide manifesting as a decrease in signal intensity on T2∗ MRI. In malignant lymph nodes, however, tumor cells displace normal sinus macrophages, leading to reduced USPIO uptake and maintenance of high signal on T2∗ MRI. Lymphotropic nanoparticle-enhanced MRI (LNMRI) provides a way to distinguish malignant versus benign lymph nodes during cancer staging, which is often difficult based on size criteria alone.
Magnetic Resonance Spectroscopy
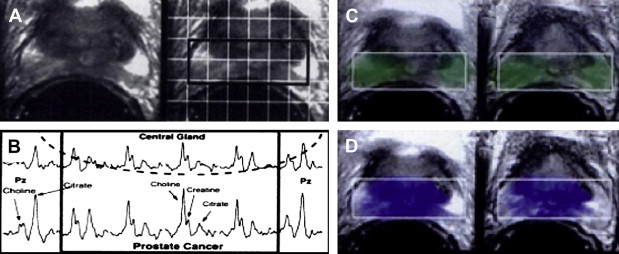
Magnetic resonance spectroscopy (MRS) combines conventional MRI anatomic imaging with spectroscopic evaluation of cellular metabolites. In the prostate, the important metabolites are choline and citrate. Choline is an important component of cell membranes, integrated into the phospholipid bilayer. Prostate malignancy is hypothesized to lead to increased choline because of increased cell proliferation. Citrate is a component of the citric acid cycle that normally accumulates within the glandular ducts formed by prostate epithelial cells. Prostate malignancy is thought to lead to decreased choline levels by means of increased tumor metabolic activity and decreased glandular differentiation. High-resolution, three-dimensional, quantitative proton spectroscopy can be performed using endorectal surface coils and volumetric spectroscopic sequences.
Optical Imaging
Optical imaging measures light photons of a given wavelength spectrum transmitted through tissues. Optical techniques generate useful images from tissues by exploiting endogenous differences in tissue contrast or through the administration of optical probes designed to increase target tissue photon transmission. Optical coherence tomography (OCT) takes advantage of different light optical backscattering properties among cell layers to create real-time optical scattering images of tissue microarchitecture with a spatial resolution on the order of 10 μm. Although originally conceived for ophthalmologic use because of limited tissue penetration (2–3 mm), the recent development of endoscopic OCT probes has made minimally invasive evaluation of microstructure in other epithelialized tissues possible. Most exogenously administered optical probes being considered for human molecular imaging applications are fluorescent probes consisting of a molecularly targeted agent conjugated with a fluorophore that absorbs and emits light within a defined spectral range. Fluorescent probes generate high contrast levels because of low background tissue autofluorescence but are often limited by poor transmission of emitted light through tissue, precluding imaging of deep structures. Near-infrared fluorescence (NIRF) imaging uses fluorescent probes in the near-infrared range (640–900 nm) that can transmit light through tissues easily with minimal absorption by water and hemoglobin. NIRF probes can be imaged up to 10 cm deep within the body and can be combined with tomographic imaging techniques to generate three-dimensional quantitative determination of probe distribution in vivo. Additional advantages of NIRF optical imaging include lack of ionizing radiation exposure to patients and the ability to image multiple probes simultaneously.
Applications
Positron emission tomography of testicular germ cell tumors
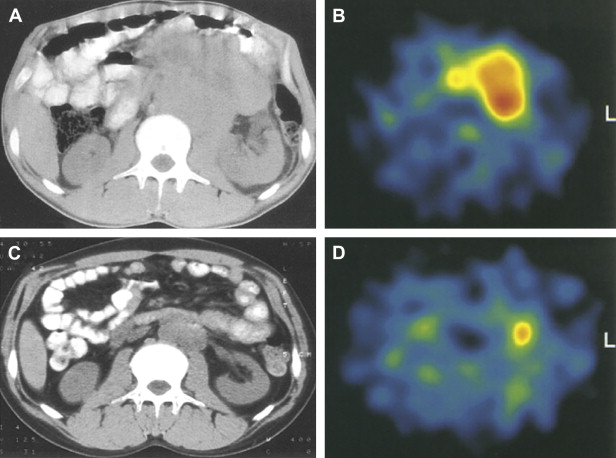
A 1995 study examining FDG-PET imaging of 11 who had with testicular germ cell tumors (GCTs) demonstrated increased tracer uptake within the tumors and their metastases relative to fibrotic regions, normal tissues, and mature teratomas ( Fig. 1 ). A 1999 study comparing FDG and CT for initial staging in 37 patients who had testicular GCTs showed that FDG-PET was superior to CT for detection of lymphatic and distant metastases and correctly staged disease in 92% of cases compared with 68% for CT. A 2004 study directly compared FDG-PET and CT for assessing viable residual tumor in 51 patients who had metastatic seminoma and were undergoing chemotherapy. Correlation with histology demonstrated FDG-PET to be superior to CT for detection of residual malignancy, with 100% specificity and 80% sensitivity compared with 70% and 74%, respectively, for CT.
Lymphotropic Nanoparticle-Enhanced MRI for Lymph Node Metastasis
Prostate cancer
A study of 80 patients who had resectable prostate cancer (T1–T3) undergoing LNMRI with USPIO (Ferumoxtran-10) followed by histologic lymph node sampling demonstrated 91% sensitivity and 98% specificity for LNMRI on a node-by-node basis ( Fig. 2 ). The sensitivity of LNMRI for lymph node metastasis was statistically increased compared with conventional MRI. Within the subset of smaller lymph node metastases (5-10 mm in short-axis diameter) that would be considered normal on conventional CT or MRI, LNMRI demonstrated 96.4% sensitivity for metastasis. A 2008 study of 60 patients who had prostate cancer undergoing LNMRI also examined whether USPIO could detect the primary prostate malignancy. This study found a statistically significant association between the magnitude of the decrease in prostate gland T2∗ intensity after USPIO administration and the histologic tumor grade for intermediate- and high-grade prostate malignancies. In this case, the USPIO are thought to be taken up by infiltrating macrophages within the prostate tumor and surrounding stroma.

Bladder cancer
Identification of lymphatic metastasis in patients who have muscle invasive bladder cancer is important to identify patients likely to require adjuvant chemotherapy. Accurate noninvasive determination of pelvic lymphatic metastasis before surgery would be a useful surgical guide for resection of lymphatic metastases and would potentially help to reduce complications associated with extensive pelvic lymphadenectomy. A study of 58 patients who had bladder cancer undergoing LNMRI before surgical lymph node dissection demonstrated 96% sensitivity and 95% specificity compared with histology (n = 172 lymph nodes). This study included 9 patients in whom LNMRI identified metastatic lymph nodes outside of the surgical dissection field.
Renal cell carcinoma
The presence of lymph node metastases in RCC is known to confer decreased life expectancy. The optimal extent of lymphadenectomy accompanying surgical nephrectomy remains a topic of active investigation, however, particularly given the relatively low incidence of unsuspected lymphatic metastases and the potential morbidity of lymph node dissection compared with nephrectomy alone. A 2008 study of nine patients with renal masses who underwent USPIO LNMRI before nephrectomy and lymph node dissection showed that LNMRI demonstrated high sensitivity (100%) and specificity (95.7%) for detection of RCC lymphatic metastases. This included accurate diagnosis of a benign enlarged lymph node in one patient that would have been considered metastatic on conventional CT or MRI based on size alone. The study also demonstrated a difference in USPIO-associated reduction in T2∗ signal intensity between benign oncocytomas and RCCs (77.8 versus 5 milliseconds), suggesting a possible additional role of USPIO for discriminating benign from malignant primary renal masses.
Magnetic resonance spectroscopy of prostate cancer
Prostate adenocarcinoma has been shown to demonstrate higher choline levels and lower citrate levels compared with benign prostatic hypertrophy and normal prostate peripheral zone tissue. A 1996 study performed MRS on nine healthy volunteers, 5 patients who had BPH, and 85 patients who had biopsy-proved prostate cancer and BPH to determine whether MRS could discriminate prostate adenocarcinoma from normal prostate and BPH ( Fig. 3 ). MRS of prostate cancer demonstrated significantly lower mean citrate and higher mean choline levels compared with normal peripheral zone tissue in the same patient. A peak area ratio of [(choline + creatine)/citrate] was adopted to quantify the opposing changes in choline and citrate levels in prostate cancer into a single value. The peak area ratio was significantly elevated in prostate cancer compared with BPH or normal peripheral zone. Additionally, there was no overlap in peak area ratios between prostate cancer and BPH or normal in any of the patients imaged. These results suggest a role for MRS in detecting prostate cancer and evaluating its spatial distribution.