For clinically localized prostate cancer, radical prostatectomy remains the “gold standard” treatment. New forms of minimally invasive therapies are sought out by patients, however, because of the potential morbidity associated with open surgery. With quality-of-life aspects influencing patient decision making, minimally invasive therapeutic modalities have generated great interest among patients. Laparoscopic radical prostatectomy, robotic-assisted laparoscopic prostatectomy, brachytherapy, cryotherapy, and high-intensity focused ultrasound are all considered to be minimally invasive treatment options for the management of clinically localized prostate cancer.
Prostate cancer is the second most common cancer in men in the United States. It has been estimated that 218,890 men would be diagnosed with prostate cancer in 2007 and 27,050 would die from this disease . Prostate-specific antigen (PSA) screening, which started in the 1980s, has brought about a significant increase in the detection of prostate cancers at an earlier stage. Urologists are now frequently treating younger men with less advanced disease who have excellent preoperative urinary and sexual function. Today, if a patient is diagnosed with prostate cancer, he is more likely to have clinically nonpalpable disease than his counterparts in the pre-PSA era.
For clinically localized prostate cancer, radical prostatectomy remains the “gold standard” treatment. New forms of minimally invasive therapies are sought out by patients, however, because of the potential morbidity associated with open surgery. With quality-of-life aspects influencing patient decision making, minimally invasive therapeutic modalities have generated great interest among patients. Laparoscopic radical prostatectomy (LRP), robotic-assisted laparoscopic prostatectomy (RALP), brachytherapy, cryotherapy, and high-intensity focused ultrasound (HIFU) are all considered to be minimally invasive treatment options for the management of clinically localized prostate cancer.
Laparoscopic radical prostatectomy
The first successful LRP in a man was performed in 1997 . This initial experience was characterized by long operative times (8–11 hours) and an average hospitalization of 7.3 days. Based on a limited series of nine patients, it was initially believed that LRP provided no significant benefit compared with open radical prostatectomy for treatment of clinically localized prostate cancer. Widespread acceptance and application of LRP was made possible by the efforts of French urologists who published their experience and early results with this technique in 2000 . These results demonstrated reduced operative times (4–5 hours per case), acceptable continence rates after surgery (72%–84%), and preservation of erections in approximately 45% of patients with a sufficient level of preoperative sexual function.
Patient selection
Indications for LRP are identical to those for open radical prostatectomy: patients who have clinically localized prostate cancer with no evidence of metastatic disease and are in sufficiently good health to undergo surgery. There are relatively few contraindications to LRP; however, the presence of uncorrectable bleeding diatheses or inability to tolerate general anesthesia or cardiopulmonary effects of laparoscopy may warrant pursuit of alternative treatment methods. LRP can be safely performed in patients who have a history of prior abdominal surgery, hormonal therapy, or morbid obesity . The primary goals of LRP remain the same as those of open radical prostatectomy: (1) cancer control, (2) preservation of urinary continence, and (3) preservation of erectile function.
Transperitoneal technique
Anatomic principles and surgical dissection of the prostate are similar regardless of whether a pure laparoscopic or robotic-assisted approach is used. LRP is performed under general anesthesia with the patient in a supine position. The patient is secured to the table at the shoulder level to prevent movement when placed in a steep Trendelenburg position during a transperitoneal approach. LRP can also be performed in an extraperitoneal fashion; in that case, the patient can remain in a more neutral position. The legs are slightly spread apart to permit access to the perineum during the operation. A Foley catheter is inserted at the beginning of the procedure, and a nasogastric tube is used to decompress the stomach.
Pneumoperitoneum can be established with the use of a Veress needle and the abdominal cavity entered under direct vision through a periumbilical incision using a Visiport device. Additional trocars are placed inferiorly and laterally for passage of laparoscopic instrumentation ( Fig. 1 ). Once all trocars have been inserted and the patient has been positioned in a steep Trendelenburg position to facilitate retraction of the small bowel out of the operative field, a transverse incision is made in the peritoneum below the base of the bladder in the region in which the vas deferens joins the seminal vesicles. The seminal vesicles are dissected free from surrounding tissues, and vascular pedicles are ligated with titanium or Hemolockclips ( Movie 1: Dissection of seminal vesicles and vas deferentia ∗
∗ Videos for this article can be accessed by visiting www.urologic.theclinics.com . In the online table of contents for this issue, click on “add-ons.”
). Every attempt is made to avoid the use of electrocautery during lateral dissection of the seminal vesicles to prevent thermal injury to the nearby cavernous nerves responsible for erections.
Once the seminal vesicles have been mobilized, a transverse incision is made in Denonvilliers’ fascia to dissect the plane between the prostate and rectum ( Movie 2: Posterior prostatic dissection ∗ ) ( Fig. 2 ). To maximize neurovascular bundle preservation, a closer dissection to the prostate posteriorly can be performed that leaves Denonvilliers’ fascia on top of the perirectal fat. Alternatively, a deeper plane of dissection between Denonvilliers’ fascia and perirectal fat can be performed when more extensive or palpable cancer is present. Once the plane between the prostate and rectum has been developed, attention is then turned to the anterior prostatic dissection.

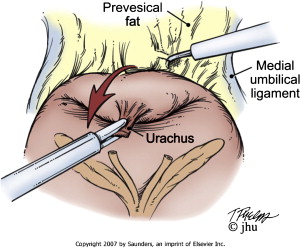
The space of Retzius is entered transperitoneally by dividing the urachus above the bladder and incising the peritoneum just medial to the medial umbilical ligaments ( Movie 3: Developing the space of Retzius ∗ ) ( Fig. 3 ). Mobilization of the bladder is performed, and the alveolar tissue anterior to the bladder within the extraperitoneal space is dissected to expose the prostate. The endopelvic fascia and puboprostatic ligaments are sharply divided to mobilize the prostate and expose the levator muscle fibers. The deep dorsal venous complex (DVC) can be suture ligated for hemostasis ( Movie 4: Ligation of the deep dorsal venous complex ∗ ); however, the DVC is typically divided later during the operation before the apical dissection of the prostate and division of the urethra ( Movie 5: Division of deep dorsal venous complex ∗ ).
At this point in the operation, a transverse incision is made in the anterior bladder neck ( Movie 6: Bladder neck transection and prostatic pedicle ligation ∗ ). This plane of dissection is carried through the posterior bladder neck to expose the previously dissected seminal vesicles. The seminal vesicles can then be pulled through this working space to provide mobilization of the prostate for the remainder of the operation ( Fig. 4 ). The vascular pedicles to the prostate can be ligated with titanium or Hemolock clips to avoid the use of electrocautery, which may damage the neurovascular bundles ( Movie 7: Antegrade neurovascular bundle preservation ∗ ). Once the neurovascular bundles have been dissected away from the prostate, the DVC and urethra can be transected from the apex of the prostate ( Movie 8: Prostatic apical dissection and division of urethra ∗ ). The vesicourethral anastomosis may be accomplished using an interrupted closure or a running continuous suture with a single knot ( Movie 9: Interrupted vesicourethral anastomosis ∗ ) . An anterior or posterior tennis racquet closure of the bladder neck may be required if there is a significantly sized discrepancy in the bladder neck urethral opening.

Extraperitoneal technique
LRP can also be performed by means of an extraperitoneal approach. An infraumbilical incision is made, and the space of Retzius is entered under direct visualization with a Visiport device. The trocar and camera are removed, and the extraperitoneal space is developed using a trocar-mounted balloon dilator device ( Movie 10: Extraperitoneal baloon access ∗ ) ( Fig. 5 ). The balloon is inserted into the preperitoneal space and insufflated under direct vision. Additional trocars are inserted, and transection of the bladder neck occurs as one of the initial steps in this surgical approach. The remainder of the operation is similar to the transperitoneal approach.
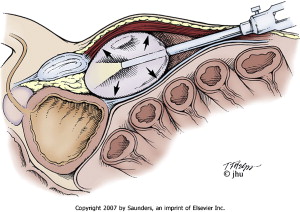
Some studies have reported improved outcomes for extraperitoneal LRP compared with the transperitoneal approach. Extraperitoneal LRP has been associated with less operative time and has enabled faster recovery of continence compared with the transperitoneal approach . Other groups have found no significant differences in clinicopathologic outcomes between the two techniques .
Pelvic lymphadenectomy
Laparoscopic pelvic lymphadenectomy may be performed in patients when indicated (ie, Gleason score ≥7, palpable disease, preoperative PSA ≥10). Laparoscopic PLND can be performed by the transperitoneal or extraperitoneal approach and using a pure laparoscopic technique or with robot assistance ( Movie 11: Laparoscopic pelvic lymph node dissection ∗ ). The same limits of lymphadenectomy are respected as with open surgery, including the external iliac vein, pubis, obturator nerve, and bifurcation of the iliac vessels. Thermal energy and sharp dissection should be minimized to avoid vascular and neural injury. Clips should be applied to lymphatic channels to prevent postoperative lymphocele.
Pathologic outcomes after laparoscopic radical prostatectomy
The primary goal of radical prostatectomy is complete removal of the entire prostate. The most common site of a positive margin during LRP is at the prostatic apex . Positive margin rates for pT2 and pT3 disease have been reported to range from 4.7% to 18.4% and from 26.2% to 45.7%, respectively . Increasing experience has been shown to be associated with a decrease in the rate of positive surgical margins, suggesting that inexperience with the laparoscopic surgical approach may contribute to a higher rate of positive surgical margins.
A recent prospective analysis of clinical and pathologic outcomes of 508 men who underwent LRP at Johns Hopkins Medical Institutions demonstrated positive margin rates of 8.2% for pT2 disease and 39.3% for pT3 disease . Three-year actuarial biochemical recurrence-free survival was 98.2% for pT2N0/Nx disease, 78.7% for pT3N0/Nx/N1 disease, and 94.5% overall.
Potency after laparoscopic radical prostatectomy
Avoidance of energy sources, such as monopolar or bipolar electrocautery and harmonic scalpel, during dissection of the neurovascular bundles has been associated with improved return of potency after surgery . Potency rates after LRP have been reported to range from 46% to 88% ( Table 1 ). Hemolock clips can be used to ligate vessels during dissection of the neurovascular bundles to minimize or eliminate the use of thermal energy.
| Reference | Total patients | Evaluable patients | Definition used | Method of assessment | Assessment time | Potency rate |
|---|---|---|---|---|---|---|
| Hoznek et al | 200 | 82 | Intercourse | Questionnaire | 1 month | 46% |
| Turk et al | 125 | 44 | Intercourse | Physician | 12 months | 59% |
| Salomon et al | 235 | 43 | Intercourse | Questionnaire | 12 months | 58.8% |
| Eden et al | 100 | 100 | Erections | Physician | 12 months | 62% |
| Guillonneau et al | 550 | 47 | Intercourse | Physician | 1.5 months | 66% |
| Anastasiadis et al | 230 | 230 | Intercourse | Questionnaire | 12 months | 53% |
| Roumeguere et al | 85 | 85 | Intercourse | Questionnaire | 12 months | 65.3% |
| Stolzenburg et al | 700 | 185 | Intercourse | Questionnaire | 6 months | 47% |
| Rassweiler et al | 5824 | NA | Intercourse | Questionnaire | 12 months | 52.5% |
| Su et al | 177 | 177 | Intercourse | Questionnaire | 12 months | 76% |
| Goeman et al | 550 | NA | Intercourse | Questionnaire | 12 months | 56% |
| Gill and Ukimura | 76 | 54 | Intercourse | Questionnaire | 12 months | 88% |
A combined antegrade and retrograde laparoscopic approach for neurovascular bundle dissection with minimization of thermal energy can be accomplished with minimal blood loss and with excellent anatomic nerve preservation. The authors’ experience with this technique has demonstrated that approximately 76% of patients engaging in sexual intercourse before surgery who underwent bilateral nerve preservation reported the ability to engage in sexual intercourse 1 year after LRP . Improved potency outcomes have also been reported with a technique that incorporates temporary control of the lateral prostatic pedicle with bulldog clamps and real-time intraoperative transrectal ultrasound to visualize pulsations of the cavernous vessels within the neurovascular bundle . Among patients with excellent preoperative potency (SHIM ≥ 22), the 1-year intercourse rate when using this technique was 88%. Faster recovery of erectile function using this technique was also observed and was found to be correlated with preserved pulsatile blood vessels within the neurovascular bundle on power Doppler transrectal ultrasonography .
Continence after laparoscopic radical prostatectomy
Urinary incontinence after LRP is typically manifested by stress incontinence attributable primarily to intrinsic sphincter deficiency. Differences in surgical technique may account for variability in the levels of continence after LRP; however, the exact physiologic mechanisms that contribute to urinary control after surgery are not entirely understood. Continence rates after LRP have been reported to range from 71% to 92% at various follow-up times ( Table 2 ). It has been demonstrated that reconstruction of the posterior rhabdomyosphincter may lead to more rapid recovery of continence after radical prostatectomy . At the time of catheter removal, 74.2% of patients were continent using this reconstructive technique after LRP .
| Reference | Total patients | Definition used | Method of assessment | Follow-up (months) | Continence rate |
|---|---|---|---|---|---|
| Hoznek et al | 200 | No pad | Questionnaire | 12 | 86% |
| Turk et al | 125 | 0–1 pad | Physician | 9 | 92% |
| Olsson et al | 228 | No pad | Questionnaire | 12 | 78.4% |
| Salomon et al | 235 | No pad | Questionnaire | 12 | 90% |
| Eden | 100 | No pad | Physician | 12 | 90% |
| Guillonneau et al | 550 | No pad | Physician | 12 | 82.3% |
| Anastasiadis et al | 230 | No pad | Questionnaire | 12 | 71.6% |
| Roumeguere et al | 85 | No pad | Questionnaire | 12 | 80.7% |
| Stolzenburg et al | 700 | No pad | Questionnaire | 12 | 92% |
| Rassweiler et al | 5824 | No pad | Questionnaire | 12 | 84.9% |
| Goeman et al | 550 | No pad | Questionnaire | 12 | 82.9% |
Evolution of robotic surgery
In 1495, Leonardo da Vinci designed a “mechanized” mannequin in the form of an armed knight. During the Renaissance, this became a model from which numerous other mannequins were constructed for entertainment . The term robot was first coined by Karel Capek in the 1923 book, Rossum’s Universal Robots and relates to the Czech word for slave labor, robota . The word robot and its practical applications increased only in the twentieth and twenty-first centuries, however.
The earliest uses of robots were for military purposes. The first surgical application was in 1985 in a neurosurgical procedure . It was used to orient a needle for a brain biopsy under CT guidance. In 1992, International Business Machines (IBM) and associates developed a prototype for orthopedic surgery. The “ROBODOC” was used to assist surgeons in milling out a hole in the femur for total hip replacements . A new era was beginning, and the concept of telepresence technology, which would allow the surgeon to operate at a distance from the operating room, was being intensively researched simultaneously at the Stanford Research Institute, Department of Defense, and National Aeronautics and Space Administration (NASA). Intuitive Surgical acquired the prototype and commercialized the system. Soon thereafter, Computer Motion unveiled the first laparoscopic camera holder. Computer Motion later created a surgical system, which is an integrated robotic system . In March 2003, the fusion of both companies was announced under the name of Intuitive Surgical, Inc.
The Intuitive Surgical system’s main components are a control console that is operated by the surgeon and the surgical cart, which consists of four arms. The arms are operated by manipulation of two master controls on the surgeon console. The Intuitive Surgical system has tremor filtration, movement scaling, increased range of motion, three-dimensional vision, and ergonomic advantages. All these features make it ideal for complex laparoscopic movements in an anatomically confined space.
Binder and Kramer from Germany reported on the first RALP in 2001. In 2002, a team from Henry Ford Hospital in Detroit reported on their initial experience with the use of the Intuitive Surgical robot . Since then, a tremendous growth has been seen all around the world in adoption of this technique. In the year 2007, it was estimated that more than 40,000 radical prostatectomies would be performed robotically (personal communication, Intuitive Surgical).
Evolution of robotic surgery
In 1495, Leonardo da Vinci designed a “mechanized” mannequin in the form of an armed knight. During the Renaissance, this became a model from which numerous other mannequins were constructed for entertainment . The term robot was first coined by Karel Capek in the 1923 book, Rossum’s Universal Robots and relates to the Czech word for slave labor, robota . The word robot and its practical applications increased only in the twentieth and twenty-first centuries, however.
The earliest uses of robots were for military purposes. The first surgical application was in 1985 in a neurosurgical procedure . It was used to orient a needle for a brain biopsy under CT guidance. In 1992, International Business Machines (IBM) and associates developed a prototype for orthopedic surgery. The “ROBODOC” was used to assist surgeons in milling out a hole in the femur for total hip replacements . A new era was beginning, and the concept of telepresence technology, which would allow the surgeon to operate at a distance from the operating room, was being intensively researched simultaneously at the Stanford Research Institute, Department of Defense, and National Aeronautics and Space Administration (NASA). Intuitive Surgical acquired the prototype and commercialized the system. Soon thereafter, Computer Motion unveiled the first laparoscopic camera holder. Computer Motion later created a surgical system, which is an integrated robotic system . In March 2003, the fusion of both companies was announced under the name of Intuitive Surgical, Inc.
The Intuitive Surgical system’s main components are a control console that is operated by the surgeon and the surgical cart, which consists of four arms. The arms are operated by manipulation of two master controls on the surgeon console. The Intuitive Surgical system has tremor filtration, movement scaling, increased range of motion, three-dimensional vision, and ergonomic advantages. All these features make it ideal for complex laparoscopic movements in an anatomically confined space.
Binder and Kramer from Germany reported on the first RALP in 2001. In 2002, a team from Henry Ford Hospital in Detroit reported on their initial experience with the use of the Intuitive Surgical robot . Since then, a tremendous growth has been seen all around the world in adoption of this technique. In the year 2007, it was estimated that more than 40,000 radical prostatectomies would be performed robotically (personal communication, Intuitive Surgical).
Robotic-assisted laparoscopic prostatectomy
Similar to LRP, RALP can be performed transperitoneally or extraperitoneally. Pneumoperitoneum is generated using a Veress needle or Hasson technique. The abdomen is insufflated using carbon dioxide at 15 mm Hg, and trocars are placed under direct vision. The patient is placed in a lithotomy position and in a steep Trendelenburg position. The robot is docked to the trocars, and the procedure is begun using a 0° binocular lens, monopolar scissors in the right arm, PK dissecting forceps in the left arm, and the Prograsp in the fourth arm.
The anterior peritoneum is incised (bladder takedown) to enter the retropubic space of Retzius. The endopelvic fascia is then incised, and the levator ani fibers are separated from the prostate. The DVC is ligated, and a suspension stitch is placed with a 1-0 Monocryl suture on a CT-1 needle.
To proceed with the bladder neck dissection, a scope with a 30° down angle is used. Dissection proceeds in a downward direction until the urethra and catheter are visualized. The posterior wall of the bladder neck is dissected, and the vas deferens and the seminal vesicles are exposed. The seminal vesicle is dissected by approaching its medial wall initially in an atraumatic and athermal fashion.
Denonvilliers’ fascia is then incised, and the posterior rectal plane is developed distally toward the urethra. The pedicles are ligated with hemostatic clips, and an athermal early retrograde release of the neurovascular bundle is performed.
Apical dissection is then performed using cold scissors to divide the DVC and urethra. The vesicourethral anastomosis is performed using a modified technique by Van Velthoven and colleagues . A single continuous running suture is used by tying two separate 20-cm length 3-0 Monocryl sutures together with 10 knots. The posterior anastomosis is performed with one arm of the suture beginning at the 5 o’clock position running clockwise to the 10 o’clock position. The anterior anastomosis is completed with the second arm of the suture starting in the 5 o’clock position and proceeding in a counterclockwise fashion. Both sutures are tied in the 10 o’clock position on the urethral stump. A Foley catheter is left in place for 4 to 7 days. As with LRP, pelvic lymphadenectomy may be performed using robotic assistance when indicated.
Operative outcomes
Operative time
Comparing operative times is difficult in large series because they include setup time and pelvic lymph node dissection ( Table 3 ). Operative times may also vary according to the learning curve. The operative time decreases as surgeon experience grows. In a previously reported series by Patel and colleagues , mean operative time was 130 minutes in their first 500 cases. With the authors’ experience now having passed 1500 cases, the operative time has been reduced to 90 minutes . This decrease in time has been noted despite adding a few new surgical variations to the authors’ previously described technique (ie, DVC and urethral suspension, posterior rhabdosphincter reconstruction). The mean operative time reported by Menon and colleagues in 2002 was 274 minutes in their first 40 patients. In recently published data by the same investigators, the mean operative time reported was 154 minutes .








