Chapter 81A Metastatic malignant liver tumors
Colorectal cancer
Overview
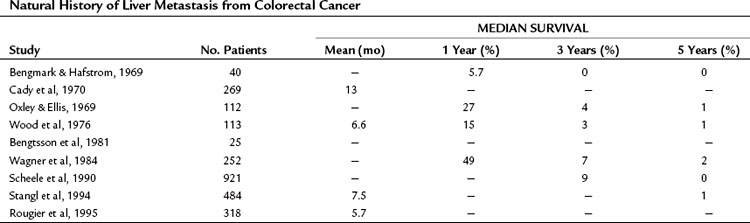
The liver is the most common site for hematogenous metastasis from colorectal cancers (CRCs). One quarter of patients with primary colorectal carcinoma present with synchronous hepatic metastasis, and nearly half of the patients resected of their colorectal primary will eventually develop metachronous liver metastasis (Bozzetti et al, 1987; Ekberg et al, 1987). In patients with isolated hepatic metastases, the extent of liver disease is the principal determinant of survival, and when left untreated, survival is measured in months (Norstein & Silen, 1997). Despite recent improvements in regional therapies, systemic chemotherapies, and biologic agents, survival is rarely over 3 years in the absence of surgical intervention (Table 81A.1).
Over the past 3 decades, surgery has been clearly demonstrated to be a safe and potentially curative modality in the treatment of colorectal metastases to the liver. The current 5-year survival following a margin-negative hepatic resection is 40%, with a 10-year survival approaching 20%. Although no prospective randomized trials comparing liver resection to systemic, regional, or other local therapies have been performed, the outcome for patients after liver resection for metastatic CRC is sufficiently favorable that surgery is now considered the primary therapy in selected patients (Pawlik & Choti, 2007; Ito et al, 2010).
Natural History of Metastases From Colorectal Cancer
Invasive CRC principally spreads through two mechanisms: cancer cells can metastasize to regional lymph nodes and then through central lymphatics into the systemic circulation, or they can spread directly to the liver via portal venous drainage (Knosel et al, 2004). This has been tested experimentally for CRC. In one study, investigators measured circulating transcripts of guanylyl cyclase C as a surrogate marker for circulating tumor cells and detected these two mechanisms at work in patients. During surgery for stage I or stage II primary CRC, circulating cancer cells were more frequently detectable in the portal blood as opposed to in the periphery, suggesting that the portal route may be more important in early stage patients; however, in some patients with documented lymph node spread, circulating cells were evident in peripheral blood samples but not in the portal blood (Tien et al, 2002).
When cancer spreads through the portal system, the liver acts effectively as a filter and minimizes or even prevents spread to distant sites (Picardo et al, 1998; Wang et al, 2004). At a cellular level, this process has been imaged through the labeling of cancer cells with green fluorescent protein, transplanting them into the ascending colon in mice. In this model, metastatic foci in the liver emerge within days after transplantation (Wang et al, 2004). The importance of portal spread is further validated by clinical data. Liver metastases were not observed in patients with cirrhosis (0 of 40 patients) but were observed in patients without cirrhosis (46 of 210, P < .001) in a Japanese study. The authors speculated that decreased portal flow to liver parenchyma in cirrhotic patients accounted for this phenomenon (Uetsuji et al, 1992).
It is unclear why some cancers spread via lymphatics and others spread via the portal circulation, although some theories based on experimental data have been suggested. One study found that in colon primaries, tumors located on the mestenteric side of the colon had a higher rate of lymph node spread than those on the antimesenteric side (207 cases, 51% vs. 30%; P = .004), probably related to the distribution and density of lymphatic vessels in the mesocolon (Boni et al, 2007). A separate group analyzed chromosomal copy number in primary and metastatic cancer foci using comparative genomic hybridization and found that certain reproducible allelic losses or gains were observed in hematogenous deposits, as compared with lymph node metastases.
The outcome of untreated metastatic CRC to the liver has been well documented in the older metastatic CRC literature and has been summarized in detail elsewhere (Norstein & Silen, 1997). The median survival of untreated CRC with synchronous liver metastases is just 5 to 10 months, and 3-year survival is very unusual. Survival at 5 years is essentially limited to patients who are seen initially with solitary liver metastases.
The extent of liver disease is an important determinant of prognosis. In a highly cited retrospective study by Wood and colleagues, 113 patients from the Glasgow Royal Infirmary with stage IV CRC were followed. The 1-year survival rate was 5.7% in the setting of widespread liver disease, 27% for those with metastases localized to a single segment or lobe of the liver, and 60% for patients with a solitary metastasis (Norstein & Silen, 1997). The patients with solitary metastases had a 3-year survival of 13% and a median survival of 17 months. A similar study by Wagner and colleagues (1984) found that 20% of patients who had an unresected solitary liver lesion lived 3 years.
The authors of these two retrospective natural history studies attempted to distinguish potentially resectable disease from unresectable disease and determined that the survival was substantially better in the former. In the study by Wood and colleagues (1976), 13 patients were believed to have had resectable disease; the 1-, 3-, and 5-year survival of these untreated patients were 77%, 23%, and 8%, respectively, compared with 15%, 0%, and 0% for the unresectable group. Similarly, Wagner and colleagues (1984) reported 3- and 5-year survivals for untreated resectable disease of 14% and 2%, respectively, compared with 4% and 0% for patients with unresectable disease. Notably, even patients with resectable, solitary liver metastases consistently have very poor long-term survival (<10%).
Results of Resection for Colorectal Liver Metastases
Long-Term Results
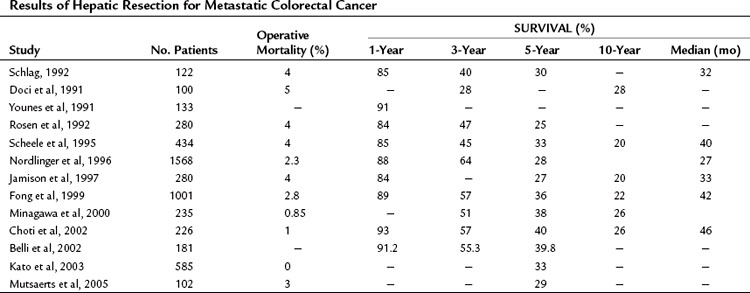
Since the studies of Wood and Wagner and colleagues, numerous large, single institutional and multicenter reports from around the world have demonstrated that liver resection is safe and results in long-term survival (Table 81A.2). In recent series, 5-year survival rates approach 40%, and median survival exceeds 40 months (Fong et al, 1999; Choti et al, 2002); this represents a dramatic improvement from the seminal study by Foster (1978) on liver resection, when the reported 5-year survival was 20%.
With newer multimodal treatments and careful patient selection, it is anticipated that 5-year survival approaching 70% can be achieved after resection (Nikfarjam et al, 2009), and comparable outcomes are likely to be reported in larger studies in the near future. Many patients with CRC at stage IV are cured by surgery. A recent review of actual 10-year survivors indicated that one third of actual 5-year survivors will eventually succumb to a cancer-related death; however, 10-year survival is tantamount to a cure in all but the rarest cases (<1%) (Tomlinson et al, 2007).
Outcome and Surgical Volume
A number of studies have correlated perioperative outcome to hospital volume for hepatectomy. Choti and colleagues (1998) and Glasgow and colleagues (1999) used the state registries in Maryland and California, respectively, and found that hepatectomy performed at a high-volume center resulted in improved outcomes. Surgery at high-volume centers was also associated with reductions in perioperative mortality, length of hospital stay, and cost. A recent study by Fong and colleagues (2005) based on a national Medicare database provides further support for superior outcomes at high-volume centers. The authors demonstrated that perioperative outcome correlated with the expertise of the institution, and the survival advantage persisted after the perioperative period. These data lend support to the concept of regionalization of hepatectomy to high-volume centers.
Medical Treatment of Metastatic Colorectal Disease to the Liver
Over the past 3 decades, the most widely used chemotherapeutic agent in the treatment of metastatic CRC has been 5-fluorouracil (5-FU), used either alone or in combination with other chemotherapies. Randomized clinical trials and meta-analyses in advanced CRC during the 1980s and 1990s established that 5-FU was best administered in combination with leucovorin (LV) and that 5-FU should be given as a continuous infusion over several days, as opposed to a series of daily bolus injections (ACCMAP, 1992; D’Angelica et al, 2002; de Gramont et al, 1997; MAGIC, 1998). Tumor response rates with the optimized 5-FU/LV dosing regimen reached 30%, with a median survival around 12 months. This response rate was a substantial improvement over what had been previously reported for 5-FU alone (10% to 15%), although the overall survival rates were similar (ACCMAP, 1992; MAGIC, 1998).
In 2000, two studies compared systemic therapy using irinotecan (CPT-11) in combination with 5-FU/LV with 5-FU/LV alone. Patients had stage IV CRC and had not received treatment for their metastatic disease (Douillard et al, 2000; Saltz et al, 2000). In the study by Saltz and colleagues, patients who recived the irinotecan-containing regimen—referred to as the IFL regimen, because it used bolus 5-FU—had a longer progression-free survival (PFS; 7.0 vs. 4.3 months), a higher response rate (39% vs. 21%), and a longer overall survival (median, 14.8 months vs. 12.6). Similar results were observed by Douillard and colleagues; in their study, irinotecan was combined with infusional 5-FU/LV (FOLFIRI). Based on these studies, combination therapy using irinotecan, 5-FU, and LV became the standard treatment for unresectable stage IV CRC. Subsequently oxaliplatin plus 5-FU/LV (FOLFOX) was accepted as second effective first-line combination regimen (de Gramont et al, 2000; Goldberg et al, 2004). In the study by de Gramont and colleagues (2000), PFS was 9.0 months versus 6.2 months with 5-FU/LV alone; the response rate was 50.7%, versus 22.3%; overall survival was 16.2 months versus 14.7 months (P = .1). In the study by Goldberg and colleagues (2004), outcomes were consistently superior with FOLFOX, including an overall survival of 19.5 months versus 15.0 months (P = .0001). This latter trial further demonstrated that FOLFOX was superior to a combination regimen consisting of irinotecan and oxaliplatin.
Two randomized controlled trials have compared FOLFOX and FOLFIRI against each other. Both trials concluded that the regimens had similar efficacy for metastatic CRC as a first-line treatment (Tournigand et al, 2004; Colucci et al, 2005). In the study by Tournigand and colleagues, patients were randomized to one of the two regimens and were crossed over to the opposite treatment arm upon progression. Overall survival was 21.5 months and 20.6 months in the FOLFIRI-first and FOLFOX-first treatment arms, respectively (P = .99). Roughly 15% of the patients in the trial became resectable during treatment and underwent metastasectomy. This was a more frequent occurrence in the FOLFIRI-first arm. In the second trial, no crossover was built into the study design, although a high proportion of patients received second-line treatment. The overall survival in the FOLFIRI and FOLFOX arms were 14 and 15 months, respectively (P = .28; Colucci et al, 2005). Secondary surgery was performed in about 5% in each group. Based on these studies, FOLFOX and FOLFIRI are widely considered to be equivelent in the metastatic setting and are generally selected according to the toxicity profile. The FOLFOX regimen is characterized by a higher rate of grade 3 and 4 neurotoxicity (30% vs. 5%) and neutropenia (44% vs. 24%). The FOLFIRI is associated with more nasuea and vomiting (20% vs. 5%), mucositis (10% vs. 0%), and alopecia (50% vs. 20%).
Falcone and colleagues (2007) explored an aggressive regimen with all three chemotherapeutic agents (FOLFOXIRI) and compared this treatment with FOLFIRI, and an increased rate of neutropenia and neurotoxicity was observed with FOLFOXIRI treatment; however, the more aggressive regimen was associated with better PFS (9.8 vs. 6.9 months), response rate (66% vs. 41%), and overall survival (22.6 vs. 16.7 months). In the FOLFOXIRI group, 15% of patients had secondary metastasectomy compared with 6% in the FOLFIRI group (P = .03). The difference was even greater when patients with liver-only disease were considered (36% vs. 12%, P = .02) (Falcone et al, 2007). In the above trials that evaluated the standard first-line combination regimens, the complete response rate was consistently around 5%.
Several small-molecule and antibody therapies have been developed that target growth factors or cell surface receptors important to CRC biology. Bevacizumab (Avastin), for instance, is a monoclonal antibody against vascular endothelial growth factor (VEGF) and has been approved in the first-line treatment of metastatic CRC in combination with 5-FU–based chemotherapy. FDA approval was a response to a study that compared bevacizumab and FOLFIRI to FOLFIRI alone (Hurwitz et al, 2004). The bevacizumab-containing regimen was associated with longer overall survival (median 20.3 vs. 15.6 months, P < .001) and a higher response rate (44.8 vs. 34.8%; P = .004). Bevacizumab was also evaluated in the context of oxaliplatin-based chemotherapy using either FOLFOX or XELOX (capecitabine and oxaliplatin; Saltz et al, 2008). Although the PFS was superior in the bevacizumab group (9.4 vs. 8.0 months; P = .0023), no improvement in overall survival was found (21.3 vs. 19.9 months; P .077). The response rates were the same in both arms (47% vs. 49%; P = .3). The pattern of increased PFS with novel biologic agents despite equivalent response rates is a common finding in trials with targeted agents. This phenomenon suggests that targeted therapies may improve PFS through stabilization of disease or perhaps by causing tumor necrosis, which is a confounding factor with conventional Response Evaluation Criteria in Solid Tumors (RECIST) (Tuma, 2006).
Cetuximab (Erbitux) and panitumumab (Vectibix) are both monoclonal antibodies against the epidermal growth factor receptor (EGFR) and are now an important part of the treatment algorithm for unresectable colorectal metastases (Cunningham et al, 2004; Jonker et al, 2007; Van Cutsem et al, 2007). Cetuximab is a chimeric monoclonal antibody approved for treatment of metastatic CRC in combination with irinotecan in patients with disease refractory to irinotecan (Cunningham et al, 2004) or as a single agent in patients who cannot tolerate irinotecan or oxaliplatin (Jonker et al, 2007).
Cunningham and colleagues (2004) established the first indication in their study, which tested the addition of cetuximab to irinotecan-based treatment in patients who were progressing and compared this strategy with cetuximab therapy alone. The response rates in the two groups were 22.9% and 10.8% (P = .007), and the times to progression were 4.1 and 1.5 months (P < .001), respectively. Overall survival was not significantly different between the two groups (8.6 vs. 6.9 months; P = .5; Cunningham et al, 2004). Jonker and colleagues demonstrated that cetuximab montherapy was superior to best supportive care in patients who were unable to take irinotecan or oxaliplatin-based therapy (Jonker et al, 2007). Overall survival was 6.1 months in the cetuximab group compared with 4.6 months in the control group (P < .001). The response rate was 8% with cetuximab, and disease stabilization occurred in 31.4% versus 10.9% in the control group (P < .05).
Panitumumab is a fully humanized monoclonal antibody and therefore appears to have a lower rate of serious infusion reactions compared with cetuximab (Van Cutsem et al, 2007). Like cetuximab, panitumumab is approved for single-agent therapy in patients who have progressed on standard chemotherapy (Van Cutsem et al, 2007). Patients on panitumumab had an 8-week PFS compared with 7.3 weeks with best supportive care (P < .0001), and it was associated with a 10% response rate; however, the overall survival was 7 months in both groups (P = .99). Panitumumab was also evaluated as part of a first-line regimen for metastatic CRC, but a benefit was not apparent in the absence of KRAS mutation profiling (Hecht et al, 2009). Hecht and colleagues (2009) compared standard FOLFOX or FOLFIRI plus bevacizumab with or without panitumumab. Interestingly, patients receiving panitumumab had significantly inferior progression-free and overall survival compared with the control arms.
Although both cetuximab and panitumumab are antibodies directed against EGFR, there is no evidence that EGFR expression influences response rate; however, there appears to be a strong treatment interaction with both medications, and KRAS is mutated in 40% of CRCs (Bos et al, 1987). A prospective trial of first-line treatment comparing FOLFIRI with and without cetuximab demonstrated prolonged PFS in patients with a wild-type KRAS in their tumors (Van Cutsem et al, 2009). Similarly, a recent trial evaluating first-line treatment using FOLFOX with and without panitumumab showed prolonged PFS in the setting of wild-type KRAS. Those patients with a KRAS mutation actually had significantly worse PFS (Douillard et al, 2010). Peeters and colleagues (2010) evaluated FOLFIRI plus panitumumab versus FOLFIRI alone in chemorefractory patients, demonstrating improved PFS with second-line chemotherapy in patients having wild-type KRAS tumors. Additionally, when administered as monotherapy as salvage treatment for metastatic CRC, EGFR inhibitors again were only found to be effective in patients with tumors with wild-type KRAS. In the two trials described earlier that compared EGFR inhibitor monotherapy with best supportive care (Jonker et al, 2007; Van Cutsem et al, 2007), cetuximab and panitumumab were associated with improvements in tumor response, PFS, and overall survival (Amado et al, 2008; Karapetis et al, 2008). Based on these studies, EGFR inhibitors are now used just in those patients with tumors that do not have a mutated KRAS gene. This paradigm, in which the genotype of a tumor dictates therapy, is likely to become even more prevalent in the future.
In summary, patients who have yet to receive treatment for metastatic CRC, even if adjuvant treatment was previously administered for the colorectal primary, have a 50% or greater chance at responding to modern systemic chemotherapy and can expect a median survival around 18 to 20 months in the absence of resection. Unresected patients, however, still seldom live more than 3 years after the diagnosis of metastatic disease. After a patient fails first-line therapy, the results are discouraging. Response rates of 20% or less are typically seen, regardless of the second-line treatment selected (Bensmaine et al, 2001; Comella et al, 2002; Cunningham et al, 1998, 2004; Falcone et al, 2007).
Prognostic Variables and Staging Systems
Characteristics of the Patient and Primary Tumor
Age has not been found to be a significant prognostic variable for long-term survival, although analyses examining the role of age are confounded by the fact that older patients are carefully selected for surgery (Cady et al, 1992; Ballantyne & Quin, 1993). The location of primary cancer has not been shown to affect outcome, as metastatic rectal and colon cancer have similar prognoses (Doci et al, 1991). On the other hand, the stage of the primary tumor is quite useful in staging metastatic disease. Patients who had positive lymph nodes associated with their primary CRC do worse, as do patients who present with synchronous liver disease. Histologic grade of the primary cancer is not a significant predictor of long-term survival in patients with metastatic disease (Doci et al, 1991; Ballantyne & Quin, 1993).
Clinical Characteristics of Liver Metastases
The most important clinical predictor of outcome related to the liver metastases themselves is the disease-free interval: the shorter the interval, the worse the prognosis (Rosen et al, 1992; Scheele et al, 1995). Other negative factors include multiple liver metastases—four lesions is a commonly used cutoff—bilateral disease, tumor size greater than 5 cm, and a markedly elevated preoperative carcinoembryonic antigen (CEA >200 ng/mL; Nordlinger et al, 1996).
The prognostic value of response to neoadjuvant chemotherapy is controversial, and opinions vary in the literature. In a study by Adam and colleagues (2004), the 5-year disease-free survival was so poor following progression during neoadjuvant chemotherapy—3% versus 20% in patients who had a response or stable disease—the authors proposed that such a finding should preclude liver resection. This phenomenon was confirmed in our own experience (Allen et al, 2003). Although other groups have not found this to be true, such studies were based on patients who received older chemotherapy regimens. Roughly half of the patients only received 5-FU, which might account for the high rate of progression (Gallagher et al, 2009; Neumann et al, 2009). With modern chemotherapy, the expected rates of progression would be less than 20% compared with 60% in the studies mentioned above that used 5-FU (Nordlinger et al, 2008).
Operative and Pathologic Characteristics of Liver Metastases
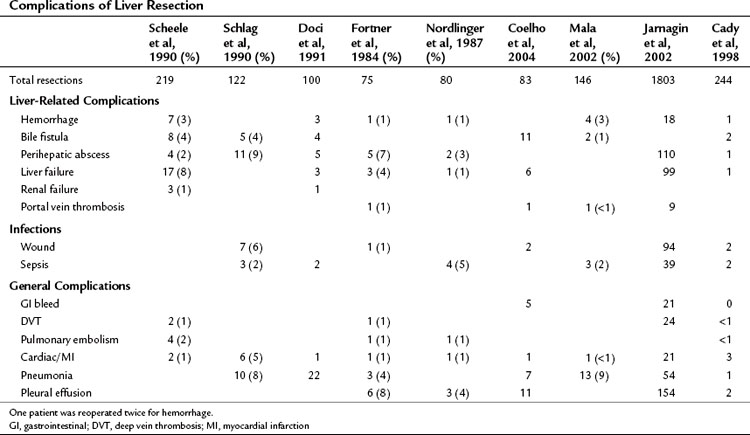
Higher operative blood loss and transfusion requirements have been found to be associated with an increased number of perioperative complications and mortality but not with a worse long-term survival (Table 81A.3; Kooby et al, 2003). Anatomic resections are believed to indirectly improve long-term outcomes, because this approach is associated with a significantly lower rate of positive margins. In a retrospective analysis from our institution, patients who had an anatomic segmental resection had a better outcome than patients subjected to wedge resections (DeMatteo et al, 2000). In an anatomic resection, planes are developed along major hepatic veins, and inadvertent disruption of the tumor is avoided. Wedge resections are usually guided by palpation and can be complicated by inadequate exposure or retraction. The attendant problems of poor visibility and impaired tactile sense at the depth of the resection will often lead to tearing of the specimen along the hard tumor–soft liver interface, thus resulting in a positive margin.
Perhaps the most informative surgical prognostic factor is the finding of extrahepatic disease (Rosen et al, 1992; Fong et al, 1999; Abdalla et al, 2006). The existence of nonpulmonary extrahepatic disease has long been considered by many surgeons to be a contraindication to hepatectomy (Fong et al, 1999; Abdalla et al, 2006); however, the approach to extrahepatic disease has been reevaluated in recent years, as some studies have demonstrated acceptable survival in selected patients with limited extrahepatic disease managed with resection, particularly in the setting of low-volume pulmonary metastases. The two largest studies on this question evaluated combination resection of liver and lung metastases and noted 5-year survival rates of 30% (Headrick et al, 2001; Miller et al, 2007).
The presence of metastatic disease to the portal lymph nodes remains a particularly poor prognostic factor. Portal metastases are found in 3% to 30% of patients with metastatic CRC to the liver (Abdalla et al, 2006), and disease recurs in virtually all patients with resected portal lymph nodes (Carpizo et al, 2009). A consensus paper on the management of extrahepatic disease concluded that no convincing data suggest that therapeutic portal lymphadenectomy for metastatic CRC improves survival (Abdalla et al, 2006). In contrast to portal lymph node metastases, there appears to be a role for hepatic resection in conjunction with metastases to other sites. The question has been examined in the form of large retrospective studies that included resections of the peritoneum or mesentery, retroperitoneal lymph nodes, and a variety of solid organs (Minagawa et al, 2000; Elias et al, 2003; Carpizo et al, 2009). The 5-year survival rates reported in these series range from 20% to 28%. Based on these studies, hepatectomy combined with surgical resection of extrahepatic disease is believed to be appropriate in selected patients.
A macroscopic positive margin, or R2 resection, is widely accepted as a poor prognostic factor. The presence of microscopic disease at the resection margin (R1 resection) or the precise thickness of the negative margin (R0 resection) is perhaps more controversial. For instance, the preponderance of data on the impact of an R1 resection suggests that a microscopically positive margin is associated with worse survival. The 5-year survivals are consistently above 30% for negative margins but are 20% or less with microscopic disease at the margin (Fong et al, 1999; Pawlik et al, 2005; Nuzzo et al, 2008); however, multivariate analyses that include R1 resection status often do not find it to be a significant predictor of outcome after adjusting for other competing risk factors, which suggests that a positive microscopic margin is merely a marker of poor biology (Pawlik et al, 2005; Nuzzo et al, 2008). As adjuvant therapy improves, patients with R1 resections will likely have outcomes that more closely approach patients with negative resection margins. Indeed, a recent report suggests that a microscopic positive resection margin obtained out of necessity does not negatively impact the 5-year survival, although the recurrence rate is increased (de Haas et al, 2008).
With regard to the extent of negative margin, a 1-cm rim of normal liver parenchyma surrounding a resected tumor was traditionally espoused in the older literature (Shirabe et al, 1997); however, recent studies suggest that subcentimeter margins are acceptable (Are et al, 2007) and are perhaps even associated with equivalent outcomes to R0 resections (Scheele et al, 1995; Pawlik et al, 2005; Hamady et al, 2006; Figueras et al, 2007). Low rates of micrometastastic disease, or satellitosis, were found in resection specimens in two Japanese studies, further supporting this idea (Yamamoto et al, 1995; Kokudo et al, 2002). Anatomic segmental resection, as opposed to wedge resections (DeMatteo et al, 2000), and neoadjuvant chemotherapy are two proposed strategies to improve R0 resection rates in patients with colorectal liver metastases (Parikh et al, 2003).
In summary, the prospect of a positive microscopic or close microscopic margin is not a contraindication to liver metastasectomy in appropriately selected patients with modern chemotherapy. In addition to a macroscopically positive resection margin, the findings of hepatic satellite lesions and intrabiliary invasion have also been shown to predict tumor recurrence (Gayowski et al, 1994; Scheele et al, 1995; Okano et al, 1999; Povoski et al, 2000; Kubo et al, 2002).
Predictive Models and Clinical Risk Scores
Four large studies have enabled robust multivariate analyses of prognostic factors with metastatic CRC and design of useful predictive models for favorable survival after metastasectomy (Nordlinger et al, 1996; Fong et al, 1999; Kattan et al, 2008; Rees et al, 2008). Nordlinger and colleagues (1996) reported on a multicenter series of more than 1500 patients. Fong and colleagues (1999) reported on a single institutional series of 1001 patients and later on a cohort of 1477 patients (Kattan et al, 2008), and Rees and colleagues (2008) evaluated long-term survival in 929 patients from a tertiary referral center in the United Kingdom.
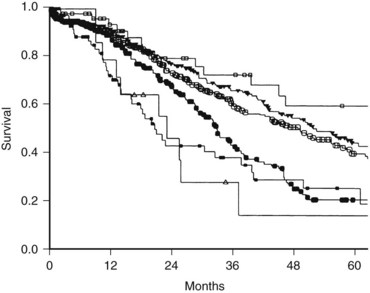
In the series by Fong and colleagues (1999), seven parameters were found to be independent predictors of an unfavorable prognosis: 1) the presence of extrahepatic disease, 2) a positive resection margin, 3) nodal metastases associated with the primary CRC, 4) a short disease-free interval, 5) largest liver metastasis greater than 5 cm, 6) more than one liver metastasis, and 7) a serum CEA greater than 200 ng/mL. The presence of extrahepatic disease and a postive resection margin are generally determined intraoperatively. When presumed preoperatively, these two variables are often considered relative contraindications to metastasectomy. A preoperative clinical risk score (CRS) system was therefore created using the last five factors (Box 81A.1), with each positive criterion counting as one point. This CRS is a simple and facile staging system used to classify patients with liver-exclusive metastatic CRC (Fig. 81A.1).
The presence of any one of these characteristics was still associated with a 5-year survival of 24% to 41%; therefore no single criteria can be considered a contraindication to resection; rather, the total score out of 5 is highly predictive of outcome, with a score of 2 or less suggestive of a particularly favorable prognosis—the optimal candidate for liver metastasectomy. Patients with a CRS of 3 or 4 have less favorable outcomes and may be appropriate for clinical trials involving adjuvant chemotherapy. Long-term disease-free survival is rare in patients with a CRS of 5, and these patients are also typically best served in clinical adjuvant chemotherapy trials. Importantly, actual 10-year survival has been observed in patients with even the highest CRS, as patients with a CRS of 3 to 5 have a 10% disease-specific survival (DSS). This is roughly equal to the cure rate of treatment in these patients; therefore a high CRS should not be considered a contraindication to hepatic resection (Tomlinson et al, 2007).
Current Use of Clinical Risk Score
The above prognostic scoring system has been validated by an independent group from Norway (Mala et al, 2002), which demonstrated that the CRS is generalizable to populations outside of the index cohort from a single, large, tertiary American center. In addition to appropriate patient selection for surgery, the CRS has proven useful in selecting patients for neoadjuvant treatment, ablation, and stratification in clinical trials (Table 81A.4).
Table 81A.4 Clinical Risk Score and Survival in Two Series on Liver Resection for Metastatic Colorectal Cancer
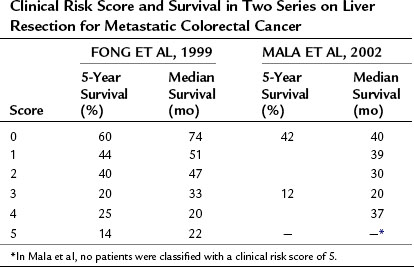
Additionally, the CRS has been shown to be useful in guiding the preoperative evaluation of a patient with metastatic CRC. As the number and cost of available tests increases, there is a growing need to develop effective ways to stratify patients so as to determine which tests are necessary for a given individual. For instance, a CRS of 1 or more was associated with a 14% rate of occult metastatic disease detectable by fluorodeoxyglucose (FDG) positron emission tomography (PET) imaging but not by conventional imaging (Schussler-Fiorenza et al, 2004). PET scanning did not have any utility in patients with a CRS of 0. Similarly, laparoscopy can be helpful for staging patients with a high CRS (>2). The finding of extrahepatic disease at laparoscopy in more than 40% of patients with elevated risk scores can prevent the added morbidity associated with an unnecessary laparotomy; however, the yield of laparoscopy is sufficiently low in the setting of a low CRS (~10% incidence of occult unresectable disease) that the added procedure is unnecessary (Jarnagin et al, 2001).
Predictive models with added sophistication have been developed recently, such as a nomogram (Kattan et al, 2008) and a multifactorial predictive index (Rees et al, 2008). Although these models are based on the same basic prognostic factors as the earlier models described above, they are more complex and difficult to use; however, as computer software and accessible handheld technology progresses, improved and more accurate predictive models will likely achieve clinical relevance in the near future.
Molecular Determinants of Outcome
Molecular profiling of CRC is an evolving area and will likely become an essential component of prediction models for cancer recurrence in the future. As stated earlier, variable response to EGFR inhibitors based on tumor KRAS status is well documented (Bos et al, 1987; Amado et al, 2008; Karapetis et al, 2008; Van Cutsem et al, 2009; Peeters et al, 2010). Similarly, response rate and the presence of microsatellite instability in the tumor are associated (Ribic et al, 2003; Nash et al, 2010). In a manner akin to the HER2 amplification and breast cancer, it is easy to imagine that these and other molecular factors will be incorporated into postoperative prognostic scales in the future.
Preoperative Investigations
Preoperative investigations prior to resection of metastatic colon cancer are directed at 1) establishing the diagnosis, 2) anatomic definition of the liver lesion for surgical planning, and 3) staging to rule out extrahepatic disease. A confirmatory biopsy of hepatic lesions is only indicated to confirm the diagnosis when the clinical picture is unclear. The differentiation between metastatic tumors and benign hepatic lesions can usually be done with imaging modalities, including ultrasound (US), magnetic resonance imaging (MRI), and PET scan as discussed below (see Chapters 13, 15, and 17). The risk of tract seeding from percutaneous fine needle aspiration appears to be quite small, with only a few case reports in the literature. The workup to determine the extent of disease includes imaging, as outlined below, and recent colonoscopy (within 6 months). Although a detailed discussion of each imaging modality is beyond the scope of this chapter, the following sections will focus on the practical aspects of imaging for preoperative workup of patients with hepatic colorectal metastases.
The Role of Preoperative Imaging
Computed Tomographic Scans
Computed tomographic (CT) scans have become indispensable in the staging of patients with metastatic CRC (see Chapter 16). A contrast-enhanced CT scan of the chest, abdomen, and pelvis are routinely obtained, although the yield from scanning the chest with regard to detecting metastatic disease is limited to just a few percent (Kronawitter et al, 1999
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree