Fig. 20.1
Standard and variant portal venous anatomy. a In standard portal venous anatomy, superior mesenteric vein ( SMV) and splenic vein ( SV) join behind the neck of the pancreas to form portal vein ( PV). At the hilum, the PV divides into right portal vein ( RPV) and left portal vein ( LPV). RPV after entering the liver substance divides into right posterior sectoral ( RPS) and right anterior sectoral ( RAS) branches. The LPV runs transversely along the base of segment 4b before turning anteriorly and caudally in the umbilical fissure where it gives branches to the segments II, III, Iva, and IVb, much less anatomical variation when compared with hepatic arterial or biliary anatomy. b The most common variations include portal trifurcation (~ 12–20 %), where RPS, RAS, and LPV share a common origin ( arrow). c Second most common variant is where RPS branch arises as a direct branch of PV (~ 9 %). In the latter situation, the LPV and right anterior PV share a common trunk ( bracket). d CT scan of a patient with the separate origin of RPS from PV. e Intraoperative image of the same patient. Notice that RAS shares a common origin with LPV
Prevention of Major Hemorrhage During Hepatic Resection
As mentioned before, a thorough understanding of the liver anatomy and detailed review of cross-sectional imaging to ascertain patient-specific anatomical variation are fundamental for safe liver resection. Over the past few decades, improvements in operative technique and advances in intraoperative and postoperative management have contributed to significant reduction in the risk of major intraoperative hemorrhage [9]. Techniques aimed at reducing blood loss during hepatic resection are discussed below.
Techniques Aimed at Reducing Blood Loss During Hepatic Surgery
One of the most important techniques shown to reduce blood loss during hepatic surgery is the use of low central venous pressure. Before the description of this technique, expansion of intravascular volume was a commonplace to provide a buffer for potential hemorrhage and hemodynamic instability. The resulting increase in central pressure is transmitted to the entire hepatic venous system; the major and accessory hepatic veins become distended and difficult to control, and there is increased back bleeding from hepatic veins during parenchymal transection. This is especially important as injury to the hepatic vein during parenchymal transection or at their junction with the vena cava is the most common cause of life-threatening intraoperative hemorrhage. With the low central venous pressure (CVP) approach, the CVP is maintained below 5 mmHg. Maintenance fluid at a low rate and hypotensive effects of the anesthetics helps in achieving the low CVP. Intermittent fluid bolus may be employed to maintain a goal urine output of 25 ml/h and systolic blood pressure greater than 90 mmHg. Maintenance of low CVP precludes vena caval distension, thus facilitating retro-hepatic dissection as well as dissection of major hepatic veins. It also reduces the bleeding during transaction of hepatic parenchyma. In case of inadvertent venous injury, the low CVP approach facilitates control of the injury. The risk of air embolism is minimized by performing the dissection in slight trendelenburg position, although this may not be necessary. The low CVP approach has been shown to reduce both the blood loss and transfusion requirements when compared to standard management [1]. Furthermore, the low CVP approach is associated with low rate of renal dysfunction when compared to total hepatic vascular exclusion.
Deliberate Dissection and Exposure of Retro-Hepatic Vena Cava and Major Hepatic Veins
We perform extensive hepatic mobilization with exposure of major hepatic veins before embarking on major hepatic resection. This provides the necessary exposure to accomplish major hepatic resection safely as well as facilitates control of veins in case of difficult intraoperative bleeding. The extrahepatic exposure and control of vein is prudent even when intrahepatic exposure and control of veins during parenchymal transaction are planned. The isolation and control of hepatic veins is especially critical when performing resection for central tumors which are close to hepatic vein-inferior vena cava (IVC) confluence as it enables adequate tumor clearance with reliable control of hemorrhage.
For right and extended right hepatectomy, control of the right hepatic vein should be achieved extrahepatically, in most cases, after the vena caval dissection has been completed. Complete division of the falciform ligament exposes the suprahepatic IVC and the right hepatic vein. Right triangular ligament is divided next to completely expose the bare area of the liver on the right. Next, the short retro-hepatic veins draining directly from the caudate lobe into the IVC are divided, progressing from the inferior aspect toward the hepatic veins (Fig. 20.2a). Complete exposure of the retrohepatic vena cava as well as the right hepatic vein requires division of vena caval ligament, which often contains of fibrous tissue but may consist of liver tissue. Care must be taken to avoid injury to the right hepatic vein or lateral wall of the IVC (see below). Division of vena caval ligament can generally be achieved using a vascular load of Endo GIA stapler. Next the tunnel between the right hepatic vein and IVC is gently developed and the right hepatic vein is encircled and controlled with vessel loop (Fig. 20.2b). No undue force should be used for any of these steps.
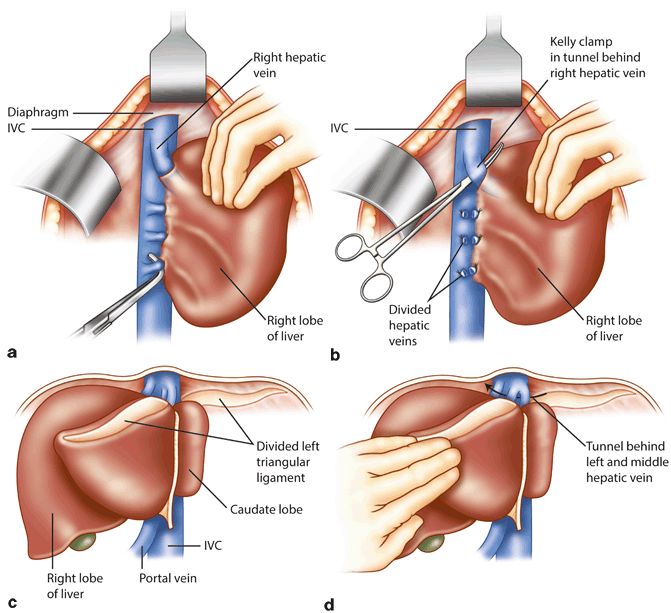

Fig. 20.2
Extensive mobilization of the liver provides access to hepatic veins and retro-hepatic IVC, which facilitates the liver resection and facilitates control of hepatic veins in case of intra-operative hemorrhage. a Division of the retro-hepatic veins draining directly into the IVC exposes the IVC. b A tunnel is developed between the IVC and right hepatic veins. If needed, right hepatic vein can be controlled with clamps. c For left-sided resections, left triangular ligament is divided and the left lobe is retracted medially. d Division of ligamentum venosum exposes the tunnel between left/middle hepatic vein and IVC
For the left and extended left hepatectomy, exposure of left and middle hepatic veins should be achieved. The left lateral sector is mobilized by division of the left triangular ligament (Fig. 20.2c). As the left triangular ligament is opened and this dissection is carried out medially, the left side of the upper part of the IVC and the left hepatic vein become visible. This dissection is carried to the right to expose the middle hepatic vein. It should be noted that, in most patients, the left and middle hepatic veins drain into the vena cava as a common trunk. Next, the ligamentum venosum is divided to achieve adequate exposure (Fig. 20.2d). The left lateral sector of the liver is turned upward and to the right, and the gastrohepatic ligament is fully divided; the ligamentum venosum, which runs in the groove between the left lateral sector and caudate lobe, is divided near its entry into the left hepatic vein. With careful dissection along the right side of the middle hepatic vein and from the left, where the ligamentum venosum attaches to the left hepatic vein, the tunnel between the left and middle hepatic veins and the IVC can be developed, allowing circumferential dissection and control of the left/medial hepatic veins. Care must be taken during this dissection in order to avoid injury to the hepatic veins (see below).
Rarely, for superiorly located tumors involving the outflow vessels near their insertion into the vena cava, control of supra- and infrahepatic IVC should be obtained preemptively (Fig. 20.3a). For infrahepatic control, an umbilical tape can be passed around the IVC below the liver but above the right renal vein (Fig. 20.3b). To control the suprahepatic IVC, division of falciform ligament and bilateral triangular ligament provides adequate exposure and the suprahepatic IVC can be dissected out and encircled (Fig. 20.3b). If difficulty is encountered in controlling the suprahepatic IVC from the abdomen, as may happen in cases of large superiorly located tumors, the incision should be extended into the right chest or vertically as median sternotomy. In such cases, persisting from the abdomen can be dangerous. Once in the mediastinum, the pericardium can be opened to get supradiaphragmatic control of IVC.
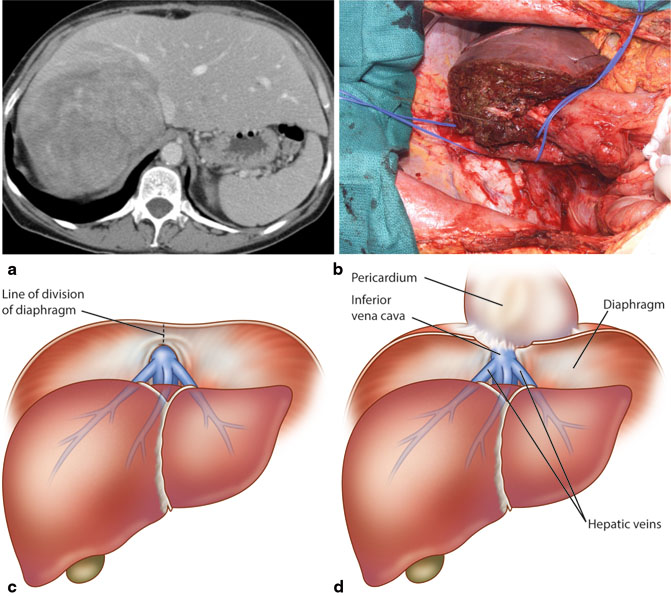

Fig. 20.3
: Preemptive control of supra- and infra-hepatic IVC should be obtained for posteriorly located large tumors. a Representative CT scan demonstrating a large hepatic tumor abutting the IVC. b Control of supra- and infra-hepatic IVC was obtained prior to proceeding with liver resection. c and d In case of massive intra-operative hemorrhage, if access to supra-diaphragmatic IVC is needed, xiphoid is resected, plane between diaphragm and pericardium is developed, and the diaphragm is divided from the xiphoid down to the IVC
Though we generally recommend extensive mobilization of liver with control of hepatic veins early in the operation, in certain situations this may not be feasible and modification of approach is necessary. This is specifically the case for large right-sided tumors that extend into the retroperitoneum and/or extensively involve the right hemi-diaphragm. In the presence of such large tumors, the mobilization of the right liver for exposure of the right hepatic vein and lateral vena cava is difficult, and attempts to do so carry a high risk of tumor disruption and significant bleeding. In such cases, anterior approach or hanging maneuver may be employed. In the anterior approach [10], hilar vessels are controlled and the liver parenchyma is transected from the anterior surface of the liver working posteriorly until the anterior surface of the right hepatic vein and IVC are encountered. The hanging maneuver [11] is a modification of anterior approach where lifting the liver with a tape passed between the anterior surface of IVC and the liver parenchyma helps to guide the resection and to control the bleeding in the depths of transection plane.
Hepatic Inflow Control
Hepatic inflow control by Pringle maneuver, which interrupts the arterial and portal venous inflow to the liver, is typically employed during hepatic resection. The lesser sac is entered by opening the lesser omentum at the level of pars flacida, finger, or blunt dissector is passed through the foramen of Winslow and the hepato-duodenal ligament containing the PV and proper hepatic artery is encircled with a vessel loop or umbilical tape. When preoperative imaging or intraoperative examination demonstrates an accessory or replaced left hepatic artery, its occlusion is needed to provide complete hepatic inflow control. The Pringle maneuver reduces inflow blood loss during hepatic resection and is generally well tolerated and does not require special anesthetic management. The major concern with hepatic inflow clamping is the ischemic injury to the hepatic remnant. This is of heightened relevance when the resection is being performed in liver with underlying cirrhosis, chemotherapy-associated steato-hepatitis, or when remnant volume is borderline. With prolonged, continuous portal venous occlusion, there is concern for splanchnic congestion with attendant consequences like bowel edema, threatened bowel anastomosis, difficult abdominal closure, increased risk of abdominal compartment syndrome, and even pancreatitis. However, these are uncommon events and can be addressed with intermittent inflow occlusion with intervals of reperfusion, which reduces hepatic ischemia as well as splanchnic congestion. We employ 10–15 min of clamping interspersed with 5 min of reperfusion. During the periods of unclamping, bleeding from the transected surface can be controlled with argon beam coagulator, clips, and/or suture ligatures. Up to 60 min of cumulative pedicle clamping time is well tolerated even with diseased liver [12].
Control of the hepatic arterial and portal venous blood supply to the portion of liver to be removed can be obtained by extrahepatic dissection or by intra-hepatic control of pedicles. This selectively interrupts the arterial and portal venous inflow to the liver to be resected and provides clear demarcation of the limits of resection. For the extrahepatic approach, we usually control the hepatic artery and PV but do not control the biliary outflow extrahepatically unless needed for tumor clearance. For right-sided resections, the right hepatic artery and PV need to be divided. To prevent inadvertent damage to the contralateral vascular inflow and troublesome bleeding, it is critical to be aware of patient’s anatomy, as up to 20 % of patients have variant right hepatic artery and nearly 20 % will have variant right portal venous anatomy. While controlling the right PV, special care should be taken to identify the posterior branch to caudate process and divide it in a controlled fashion to prevent troublesome bleeding. Extrahepatic control of inflow vessels for left-sided resection is relatively straightforward and carried out at the base of umbilical fissure.
Alternatively, the vascular pedicles can be controlled intra-hepatically and has the advantage of being rapid and unlikely to cause injury to the vascular inflow and biliary drainage of the contralateral liver. This approach is most useful for right-sided tumors located away from the hilum, which allow the use of this technique without compromising tumor clearance. The method relies on intrahepatic definition and control of the vascular pedicles. The intrahepatic pedicles are exposed after appropriate hepatotomies, dissected, encircled, and clamped. It is important to ligate the most caudal retro-hepatic veins draining from the caudate process and inferior part of the liver to the vena cava before attempting pedicular ligation. Failure to do so can result in hemorrhage during dissection of pedicles. Also, before dividing the pedicle, the integrity of vascular flow to the contralateral liver should be confirmed by ensuring good color of the remnant as well as by demonstrating the flow in the contralateral PV by intra-operative ultrasound.
Vascular Isolation
This technique is described for completeness, as it is rarely required, even during the removal of large tumor close to or involving the hepatic veins or IVC. As compared to inflow control with a Pringle maneuver, hepatic vascular exclusion combines inflow control with outflow occlusion, with or without interruption of caval flow and has more profound hemodynamic consequences. The advantage potential advantage of vascular exclusion is that the resection is performed in a relatively bloodless field; however, after flow is restored, there is often significant bleeding that requires control. Additionally, hepatic vascular exclusion is associated with 40–50 % decrease in cardiac index, 50 % increase in heart rate, 10 % decrease in mean arterial pressure, and significant increase in systemic vascular resistance. Hepatic vascular exclusion also requires prolonged continuous inflow clamping and is not well tolerated by diseased liver. Combination of inflow control with low anesthetic central venous pressure-aided resection has largely obviated the need for total hepatic exclusion.
Acute Normovolemic Hemodilution (ANH)
Acute normovolemic hemodilution (ANH) is not intended to reduce the blood loss during hepatic resection but is actually aimed at blood conservation, thus reducing the consequence of resulting blood transfusion including risk of transmitting infections as well as immunosuppression. ANH involves the removal of whole blood from a patient immediately before a liver resection that is likely to be associated with significant blood loss. After blood removal, euvolemia is restored with crystalloid infusion. Crystalloid infusion reduces the hematocrit, and thus, the blood lost during the procedure has lower hematocrit. After the completion of the procedure, or if needed during the procedure, the harvested blood with higher hematocrit than the blood lost during the procedure is transfused back into the patient. For hemodilution to be effective and feasible, the patient must have adequate starting hemoglobin, sufficient blood volume must be removed, and surgical blood volume should fall within a certain range. ANH has been shown to reduce the requirement for allogenic blood transfusion by about 50 % [13]. Despite concerns that ANH combined with significant blood loss during surgery can be potentially hazardous, multiple studies have demonstrated safety of this approach [14]. One of the major issues is the identification of patients who may benefit from the use of ANH. With improvement in surgical technique and better understanding of liver anatomy, the blood loss and the need for transfusion during and after liver surgery have decreased [9] and up to 75 % of patients undergoing major hepatectomy (> 3 segments resected) may not need allogenic blood transfusion [9]. Thus, selective application of ANH by identifying patients who have > 50 % likelihood to receive transfusion based on validated transfusion prediction model [15, 16] is both effective and resource conserving.
< div class='tao-gold-member'>
Only gold members can continue reading. Log In or Register to continue
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree








