The Royal Free Sheila Sherlock Liver Centre, Royal Free Hospital and University College London, London, UK
Hepatitis B virus (HBV) related chronic liver disease is a frequent indication for orthotopic liver transplantation (LT) in the Far East and the Mediterranean countries. Post-transplant HBV recurrence, which was almost universal in the era of no immunoprophylaxis or its short-term use, usually has an aggressive course resulting in graft loss, if left untreated [1–3]. Therefore, the HBV management is crucial for a satisfactory long-term outcome of HBV transplant patients.
HBV related liver disease was considered to be a relative or even absolute contraindication for LT in many centers, until the introduction of long-term hepatitis B immune globulin (HBIG) use in the early 1990s, which significantly decreased the post-transplant HBV recurrence rate and improved prognosis in this setting [4]. During the last decade, new antivirals, mainly nucleos(t)ide analogues, have been used, either as monotherapy or in combination with HBIG, in an effort to further improve the post-transplant outcome, treat HBIG failures, and/or reduce the need for the expensive HBIG preparations [5]. The HBV management in transplant patients can be divided into the pre-transplant, prophylactic post-transplant and therapeutic post-transplant approach [5]. In addition, the management of recipients who receive grafts from anti-HBc positive donors is discussed, as these recipients are at risk of iatro-genic HBV infection.
Pre-transplant approach
The pre-transplant approach is based on antiviral therapy in order to lower the viral load and achieve serum HBV-DNA undetectability by sensitive assays at the time of LT and thus to prevent post-transplant HBV recurrence [5]. Sometimes, the clinical improvement of HBV patients on the waiting list during or following effective antiviral therapy may even result in postponement or obviate the need for LT. Only two oral agents, lamivudine and adefo-vir dipivoxil, are currently licensed for use in patients with either chronic hepatitis B (CHB) or HBV decompensated cirrhosis, while three additional oral agents, entecavir, tel-bivudine and tenofovir disoproxil fumarate have been licensed for the treatment of CHB but not of HBV decompensated cirrhosis as yet. Interferon-alfa, which was the only available anti-HBV therapeutic option until the late 1990s, is usually contraindicated or causes intolerance and therefore cannot be used in patients with decompensated cirrhosis. The pre-transplant approach is usually combined with prophylactic post-transplant therapy [5].
Lamivudine
Lamivudine, a cytosine analogue, was the first agent that could be widely used in the pre-transplant period revolutionizing the management of such patients. Lamivudine is generally well tolerated even in severely ill cirrhotics and has a good safety profile with rare and generally mild side effects. Lamivudine, at a daily dose of 100mg, has been shown to stabilize or even improve liver function sometimes resulting in withdrawal of patients from transplant lists [6, 7]. B4 Unfortunately, the improvement or stabilization of liver function is often not sustained over time, since the prolongation of lamivudine therapy is associated with progressively increasing rates of lamivudine resistance often followed by virologic and biochemical breakthroughs [6, 7]. B4 Lamivudine resistance is usually associated with the emergence of a mutation at position 204 within the YMDD motif of the major catalytic region C of the HBV polymerase gene (substitution of methionine with valine (rtM204V) or with isoleucine (rtM204I)), often in combination with another mutation at position 180 within the region B (substitution of leucine with methionine (rtL180M)) [8]. Lamivudine resistance usually emerges after the first six months with cumulative rates of 15–25% at the end of first year and >60–65% at the end of fourth year of lamivudine therapy [9].
Breakthroughs during lamivudine therapy have a risk for severe exacerbation of liver disease, which may result in rapid development of liver failure and patient death [10]. Moreover, pre-transplant HBV viremia even when due to YMDD mutants has been associated with increased probability of post-transplant HBV recurrence [11, 12], poorer outcome after LT and lower hepatocellular carcinoma (HCC) recurrence-free post-transplant survival in patients with HCC before LT [13]. Thus, transplant centers may be reluctant to perform LT in HBV cirrhotics with viremia irrespective of the type of HBV strains [14]. Another important issue is that the emergence of lamivudine resistance may have a negative impact on the efficacy of and the probability of resistance to other anti-HBV agents [9]. For all these reasons, lamivudine monotherapy is no longer considered an appropriate first-line therapy for pre-transplant HBV patients. B4
Adefovir
The availability of adefovir, an acyclic nucleotide analogue of adenosine esterified with two pivalic acid molecules that is effective against both wild and lamivudine resistant HBV strains, was initially used in order to ameliorate the consequences of lamivudine resistance [9]. The use of adefovir monotherapy as first-line treatment in HBV-pretransplant patients has not been adequately evaluated. In CHB, adefovir monotherapy is associated with lower rates of resistance compared to lamivudine, but resistance may emerge in the second year and develop in up to 30% of cases treated with adefovir for five years [15]. Adefovir resistance is related to the emergence of a mutation at position 236 (substitution of asparagine with threonine, (rtN236T)) and/or at position 181 (substitution of alanine with valine or threonine (rtA181V or rtA181T)) of the HBV polymerase gene [16]. Another issue with adefovir monotherapy is that this agent, at least in the licensed 10 mg daily dose, is not very potent, resulting in residual viremia in approximately 80% of HBeAg-positive and 50% of HBeAg-negative CHB patients [9]. In addition, it may have some potential for nephrotoxicity. For these reasons, adefovir monotherapy was not considered as an attractive option for patients with HBV decompensated cirrhosis, in whom complete inhibition of viral replication should be achieved [17]. Because of the suboptimal profile of both lamivudine and adefovir monotherapy in HBV pre-transplant patients, ab initio use of lamivudine and adefovir combination has been recommended for such cases in several guidelines, despite the lack of strong data on efficacy and safety for such a strategy [18]. B4
Entecavir
Entecavir, a carboxylic analogue of guanosine, is a potent and selective anti-HBV agent that has been approved relatively recently for the treatment of CHB [9]. In nucleos(t) ide naive patients with HBeAg-positive or HBeAg-negative CHB, entecavir (0.5 mg daily) has greater potency than lamivudine and adefovir and a very good resistance profile with <2% cumulative five-year resistance rate [9, 19]. Entecavir was also reported to have the same good efficacy and safety profile in the subgroup of naive patients with advanced fibrosis or cirrhosis [20]. Given the absence of nephrotoxicity and its overall good safety profile in combination with the high efficacy and low resistance risk, entecavir is expected to be a first-line option for the treatment of nucleos(t)ide naive patients with HBV decompensated cirrhosis. It should be noted, however, that entecavir is not yet approved for the treatment of such patients, as relevant data are lacking.
Telbivudine
Telbivudine, another nucleoside analogue (L-deoxy-thymidine) that was approved recently for the treatment of CHB, is more potent than lamivudine but it also selects for mutations in the YMDD motif similar to those conferring lamivudine resistance (mainly rtM204I) [9]. In nucleos(t) ide naive CHB patients, telbivudine resistance starts to emerge within the first year (sixth to twelfth month) and increases during the second year of therapy. The telbivudine resistance rates were 4.4% and 2.7% at the end of first year and 21.6% and 8.6% at the end of the second year of therapy in HBeAg-positive and HBeAg-negative CHB respectively [21]. The absence of residual viremia (HBV-DNA < 400 copies/ml) at six months of telbivudine therapy is associated with low two-year probability of resistance [9]. Given its unfavourable resistance profile compared to other anti-HBV agents such as entecavir and tenofovir, the place of telbivudine monotherapy in the treatment of HBV pre-transplant patients will be unclear, even if the agent is approved for use in this setting.
Tenofovir
Tenofovir, the second acyclic nucleotide analogue of adenosine, is the most recently approved agent for the treatment of CHB. It is administered orally as tenofovir disoproxil fumarate, which is a prodrug with good oral availability and is a potent agent for the treatment of both nucleos(t)ide naive and lamivudine resistant CHB [22–24]. Tenofovir has not been licensed for treatment of decompensated cirrhosis, but there are a few case reports with successful tenofovir use in such cases [25]. However, since tenofovir may be potentially nephrotoxic, both its efficacy and safety should be tested in proper trials before its wide use in pre-transplant patients. To date, there is no evidence of resistance in CHB patients treated with tenofovir for 72 weeks, but more long-term data are lacking [22–24]. B4 Due to the great potency and the possible high genetic barrier of this agent, long-term tenofovir resistance rates are expected to be low. If its safety in decompensated cirrhosis is confirmed, tenofovir will be a first-line option for the treatment of nucleos(t)ide naive patients with HBV decompensated cirrhosis. Thus, the results of a current trial, in which tenofovir, entecavir and tenofovir plus emtricitabine are compared in patients with HBV decompensated cirrhosis, are awaited.
Management of HBV resistance
Patients with decompensated cirrhosis under antivirals should be monitored carefully for virologic response and possible virologic breakthroughs, with serum HBV-DNA testing at least every three months [18]. In CHB, any virologic breakthrough (increase in serum HBV-DNA by ≥1log10iu/ml above nadir after an early virological response) is considered in practice as viral resistance, if drug compliance is confirmed [18]. B4 In decompensated cirrhosis, however, stricter criteria of response should be applied and any detectable viremia should be considered as a treatment failure, regardless of resistance and breakthroughs, signalling the need for treatment modification.
Lamivudine resistance represents the most common problem of HBV resistance because of the longer use and the poor resistance profile of lamivudine. Adefovir was the first agent used as rescue therapy for lamivudine resistance. The addition of adefovir to ongoing lamivudine therapy instead of the substitution of lamivudine with adefovir is the preferred option for lamivudine resistant CHB [9]. In pre-transplant HBV cirrhotic patients with lamivudine resistance, the addition of adefovir results in biochemical, virologic and liver function improvement [26, 27]. In particular, in a large study with 226 HBV patients on the waiting list with lamivudine resistance, the addition of adefovir achieved HBV-DNA undetectability in 59% of cases, without adefovir resistance during the first 48 weeks [27]. B4 Moreover, alanine aminotransferase (ALT), albumin, bilirubin and prothrombin time normalized in 77%, 76%, 60% and 84% of the 226 patients respectively [27]. Despite the addition of adefovir, however, a significant proportion of patients (41% at 48 weeks and 35% at 96 weeks) maintained detectable serum HBV-DNA, which is not an acceptable result for current day practice.
Entecavir monotherapy (1 mg daily ≥2 hours before or after meals) has worse potency and higher resistance rates in lamivudine resistant than naive patients [9]. In particular, the cumulative entecavir resistance rate has been reported to be 6%, 15%, 36%, 46% and 51% at year 1, 2, 3, 4 and 5 years of entecavir therapy respectively [19]. B4 Entecavir resistance requires selection of two lamivudine resistant mutations (rtM204V/I and rtL180M) and at least one additional substitution at positions 169, 173, 184, 202 or 250 of the HBV polymerase gene (rtI169T, rtV173L, rtT184G, rtS202I or rtM250V) [28]. Thus, entecavir resistance requires three mutations in nucleoside naive patients and only one additional mutation in patients with preexisting lamivudine resistance. These findings have made entecavir a less attractive long-term therapeutic option for HBV patients with lamivudine resistance [9]. As there is cross-resistance between lamivudine and telbivudine, tel-bivudine is not expected to be effective in lamivudine resistant patients.
Tenofovir is effective against lamivudine resistant strains and is a more potent anti-HBV agent compared to adefovir [29]. Therefore, it is currently expected to become the treatment of choice for patients with lamivudine resistance. Whether switching to tenofovir or addition of tenofovir to ongoing lamivudine is the optimal and the more cost-effective approach for using tenofovir in patients with lamivudine resistance is not yet clear [29]. However, it seems safer to add in tenofovir considering the experience gained with adefovir, particularly in patients with decompensated cirrhosis.
There are no clear data for the treatment of patients with resistance to other anti-HBV agents, particularly in pre-transplant patients. Generally, resistance to an agent of one class (nucleosides: lamivudine, entecavir, telbivudine; nucleotides: adefovir, tenofovir) usually eliminates or reduces the activity of other agents of the same class due to partial or complete cross-resistance. This is well documented for lamivudine, telbivudine and entecavir, but it seems to be also the case, at least partly, for adefovir and tenofovir. Thus, in case of resistance to a nucleoside, a reasonable approach would be to add a nucleotide and vice versa. In particular in pre-transplant patients, in whom complete inhibition of HBV replication is absolutely desirable, entecavir and tenofovir (the most potent agent of each class) are expected to become the agents of choice, whenever approved. Of course, the most effective treatment of resistance is its prevention by starting treatment with agents that are potent and have a high genetic barrier [28].
Prophylactic post-transplant approach
Immunoprophylaxis alone
The efficacy of HBIG is associated with the pre-transplant type of liver disease (e.g. fulminant HBV infection, HBV and HDV co-infection) [30], viremic status and dose and duration of HBIG treatment, while the most widely accepted recommendations for HBIG prophylaxis depend mainly on the pre-transplant viremic status [31]. Patients with detectable HBV-DNA by conventional hybridization assays, who may be transplanted only after clearance of HBV-DNA by lamivudine, are treated with more aggressive HBIG protocols compared to non-viremic patients [31, 32]. B4 However, several practical questions about the ideal duration, but also about dosage, frequency and mode of HBIG administration remain to be answered [5].
HBIG may only rarely lead to eradication of HBV and therefore there is need for indefinite HBIG prophylaxis, which is an extremely expensive approach. The most cost-effective approach seems to be the individual tailoring of HBIG administration according to serum anti-HBs levels [5,31], which is time-consuming [30]. Cheaper HBIG preparations for intramuscular administration have also been tried [33–35], as they have similar pharmacoki-netic properties with intravenous preparations [36]. Interestingly, subcutaneous HBIG administration is under investigation, but no relevant long-term data are currently available [37].
Besides the high cost, long-term HBIG administration may also have some local or systemic side effects, while there is a progressively increasing risk of escape HBV mutants [8, 31]. Since the clinical significance of such HBV escape mutants has not been clarified, there is no consensus about continuation of HBIG therapy, or not, after their emergence [31], but most centers probably stop HBIG administration [5].
Another strategy aiming at cost reduction has been the substitution of HBIG with HBV vaccination. However, data on the efficacy of vaccination are rather conflicting and therefore greater numbers of patients and longer follow-up periods are required before definite conclusions can be drawn [38–42].
Prophylactic post-transplant monotherapy with nucleos(t)ides analogues
Lamivudine monotherapy, before and prophylactically after LT, gave promising short-term results [5]. However, it was subsequently shown that the efficacy of such a policy declines over time with frequent development of virologic breakthroughs and HBV recurrence in 35–50% of cases at two years post-transplant and severe clinical outcomes in some patients [43–48]. Thus, such an approach has been abandoned not only in liver transplant but also in HBsAg-positive renal allograft recipients.
There are no studies on the efficacy of monoprophylaxis with the newer nucleos(t)ides analogues. However, based on the data in pre-transplant setting, it is expected that adefovir monoprophylaxis will also be associated with emergence of adefovir-resistant mutants in post-transplant patients. Entecavir, which is a more potent agent with very low resistance rates in nucleoside naive cases and without risk for nephrotoxicity, seems to be a more attractive option for monoprophylaxis in post-transplant patients. However, entecavir should be avoided in patients with previous lamivudine resistance, which should preferably be treated with adefovir or tenofovir. Tenofovir is more potent and cheaper than adefovir having better resistance profile, but its use may also have a risk for renal dysfunction, particularly in patients receiving nephrotoxic immunosuppressive therapy. Finally, telbivudine monotherapy has moderate resistance rate and therefore does not seem to be a good option for post-transplant patients. Thus, entecavir seems the most attractive monoprophylaxis option in the post-LT HBV setting, followed by tenofovir, particularly in cases with previous lamivudine resistance. However, well-designed studies are needed to determine the optimal monoprophylaxis approach, if any. For now, the combination of HBIG (at least for a short period) and one nucleos(t) ide appears to be the most reasonable post-transplant approach, but monoprophylaxis with one of the new nucleos(t)ide analogues cannot be excluded in the future, particularly in patients with low risk of recurrence.
Prophylactic post-transplant combined approach
Post-transplant prophylactic combination of HBIG and lamivudine was tried in order to improve the efficacy of post-transplant prophylaxis and/or reduce cost. The efficacy of such a combined regimen has been shown to be superior compared to the efficacy of prophylaxis with any of the two agents alone. A recent meta-analysis of six studies showed that HBIG plus lamivudine, compared to HBIG alone, was associated with 12-fold, 12-fold and 5-fold reduction of HBV recurrence, HBV-related death and all-cause post-transplant mortality respectively [49]. Ala In 36 studies of HBIG and lamivudine combination identified in the literature, post-transplant HBV recurrence was observed in only 95 (6.3%) of 1493 patients during a mean follow-up of 6-96 months (see Table 45.1) [12,13, 32–34,48, 50–79]. It should be noted that 11 of the 95 patients with post-transplant HBV recurrence had developed YMDD mutants during pre-transplant lamivudine therapy [12, 48, 57, 64, 68, 69, 76], while HBIG and/or LAM had been discontinued when HBV recurrence occurred in another 19 patients [51, 54, 57, 71, 75]. One particularly important aspect in favor of the combined HBIG and lamivudine prophylaxis is that such an approach was used in patients with high pre-transplant viremia levels (>50% of cases had HBV-DNA detectable by hybridization assays) and achieved low HBV reinfection rates at a reduced cost. Moreover, a relatively low HBIG dosage [12, 33, 34, 58, 63, 66,67], similar to the recommended dosage for non-viremic HBV transplant patients [31], and/or intramuscular HBIG preparations were usually used [13, 33, 34, 48, 50, 51, 58, 70, 72, 75, 79], or HBIG was even discontinued after a certain period [56, 57, 69, 73]. Therefore, the prophylactic post-transplant combination of HBIG and lamivudine preceded by short-term pre-transplant anti-HBV therapy is the currently recommended approach [5]. B4 In the near future, the newer nucleos(t)ides analogues are expected to replace lamivudine in the post-transplant prophylaxis as well, probably leading to use even less HBIG.
Table 45.1 Published studies using combination of hepatitis B immune globulin (HBIG) and lamivudine (LAM) for prevention of hepatitis B virus (HBV) recurrence after liver transplantation (LT) for HBV related chronic liver disease.
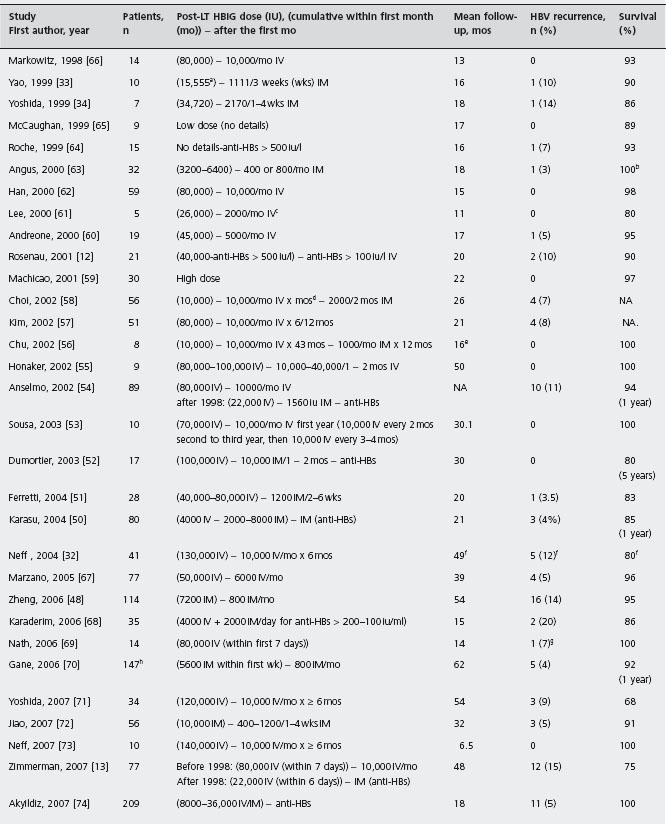
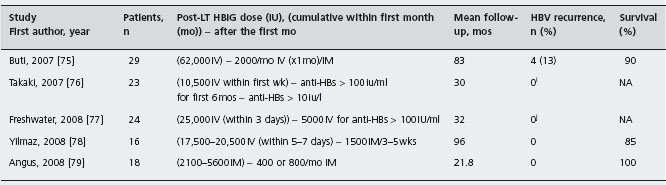
NA: not available.
aPlus 70,000IV during the first seven days in two HBV-DNA positive patients.
bFive patients, who died within one month after LT from unrelated to HBV causes, were not included in this survival estimation.
cOne patient received 80,000IU of HBIG during the first month, while another patient received only 2000IU of HBIG during the anhepatic phase and four IM injections of 650iu each within the first six months after LT.
dThirteen patients received higher HBIG dosage (10,000IU IV daily for seven days, 10,000IU IV weekly for one month, and then 10,000iuIV monthly for several years before conversion to IM HBIG.
ePost-transplant LAM was added when HBIG administration changed from IV to IM (16 months median follow-up after the onset of LAM).
fNo HBV recurrence in 18 patients with undetectable pre-LT HBV-DNA during 70 months of follow-up.
9All patients received LAM plus adefovir (10mg/day) from day 8.
hNo patient with YMDD mutants.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree







