Chapter 20 Izak Faiena, MD; Parth K. Modi, MD; Amirali H. Salmasi, MD; Allen D. Seftel, MD Laumann and colleagues from the University of Chicago reported a comprehensive study of male sexual dysfunction in 1992. The three most common male sexual dysfunctions identified were decreased libido, erectile dysfunction (ED), and ejaculatory dysfunction (EJD). The study cohort was men ages 18 to 59. Premature ejaculation (PE), a subtype of ejaculatory dysfunction, was the most common of all the male sexual dysfunctions. Sexual dysfunction was more prevalent in women (43%) versus men (31%) and was associated with age and educational attainment. In a follow-up study, Lindau and colleagues, also from the University of Chicago, conducted a large population survey. They reported the prevalence of sexual activity, behaviors, and problems in a national probability sample of 3005 U.S. adults (1550 women and 1455 men) 57 to 85 years of age, and described the association of these variables with age and health status. Among men, the most prevalent sexual problem was erectile difficulties (37%). Fourteen percent of all men reported using medication or supplements to improve sexual function. Men and women who rated their health as being poor were less likely to be sexually active and, among respondents who were sexually active, were more likely to report sexual problems. Thus there seem to be two subgroups of men with specific sexual issues based on their age. The predominant problem for the younger cohort is PE, whereas for the older cohort it is ED. In spite of these seemingly clear age-related divisions, clinically the waters have become a bit muddied. Data suggest that male ED that is vasculogenic in origin may start as early the fourth decade and that ED is related to garden-variety vascular risk factors such as hypertension, hyperlipidemia, and diabetes. Remarkably, ED that starts in the fourth decade may also be a strong predictor of future cardiovascular disease (CVD). From January 1, 1996, to December 31, 2005, a Mayo Clinic group (Inman 2009) biennially screened a random sample of 1402 community-dwelling men with regular sexual partners and without known coronary artery disease (CAD) for the presence of ED. The prevalence of ED was 2% for men aged 40 to 49 years, 6% for men aged 50 to 59 years, 17% for men aged 60 to 69 years, and 39% for men aged 70 years or older. The CAD incidence densities per 1000 person-years for men without ED in each age group were 0.94 (40 to 49 years), 5.09 (50 to 59 years), 10.72 (60 to 69 years), and 23.30 (70 years or older). For men with ED, the incidence densities of CAD for each age group were 48.52 (40 to 49 years), 27.15 (50 to 59 years), 23.97 (60 to 69 years), and 29.63 (70 years or older). These authors concluded that ED and CAD may be differing manifestations of a common underlying vascular pathology. When ED occurs in a younger man, it is associated with a marked increase in the risk of future cardiac events, whereas in older men, ED appears to be of little prognostic importance. Importantly, these researchers concluded that young men with ED may be ideal candidates for cardiovascular risk-factor screening and medical intervention. To complicate the issue a bit further, ED and male benign prostatic hyperplasia/ lower urinary tract symptoms (BPH/LUTS) have now been linked both epidemiologically and pathophysiologically, adding importance to querying for BPH/LUTS in men with sexual dysfunction, particularly ED. Thus ED is no longer easily subdivided by age into distinct groups. Significant age-related overlap exists because the importance of ED detection in the younger male appears to have prognostic importance for both BPH symptoms and CVD. This backdrop now serves as the foundation for a discussion of male sexual function and dysfunction with the hope of providing some clarity and guidance to a formerly simple disease process that has now become a bit complicated. Peyronie disease (PD) is an acquired penile deformity that manifests during a penile erection (curvature, indentation, hourglass deformity, or shortening). The condition usually presents with palpable induration (plaque) in the penis with or without painful erection. The symptomatic incidence of PD has been estimated at 1%. In white men the average age at onset is 53 years. The asymptomatic prevalence is estimated at 0.4% to 1.0%. PD has also been reported to occur in association with Dupuytren contractures, plantar fascial contractures, tympanosclerosis, trauma, urethral instrumentation, diabetes, gout, Paget disease, and the use of beta-blockers. This condition may occur in a familial pattern. PD can cause significant psychological bother and distress and potentially strain sexual relationships. Anxiety and stress manifest in a variety of ways. In a survey of 92 men with diagnosed PD, 48% were classified as clinically depressed based on their scores on the Center for Epidemiological Studies Depression Scale. In an analysis of the prevalence of depression as a function of time since diagnosis, the percentage of PD patients with depression did not change significantly in patients with PD for less than 18 months versus those who have carried the diagnosis for more than 18 months, suggesting a lack of mental adjustment to the diagnosis of PD. Similarly, in a subset of patients who were assessed for depression at baseline and after 18 months, the percentage of patients with severe depression was not statistically different (21% versus 23%, respectively). In addition to depression, men with PD also experience problems with psychosocial and sexual function. In interviews, patients with PD indicated that problems with functioning typically fall into one or more of four domains: (1) physical appearance and self-image, (2) sexual function and performance, (3) PD-related pain and discomfort, and (4) social stigmatization and isolation. While men with PD varied in the type and intensity of their subjective reactions to this condition, the major themes and patterns of response were consistent across groups. A questionnaire survey of men with PD also revealed a high prevalence of emotional and relationship problems. PD is a wound-healing disorder within the penis characterized by formation of a fibrous inelastic plaque, predominantly of collagen, on the tunica albuginea, the fibrous sheath surrounding the corpora cavernosa of the penis. The formation of fibrotic plaques results in penile deformities during erection including curvature, shortening, narrowing (hourglass), and bending (hinge effect). The degree to which PD limits sexual performance varies, depending on the angle and orientation of the penile curvature. Patients with mild curvature may feel slight discomfort during penetration, whereas patients with more severe curvature may be incapable of intercourse. Interestingly, some evidence suggests that the degree of curvature deformity does not directly correlate with the severity of the psychosocial bother of the disease. Men with lesser degrees of curvature may be as bothered as men with more severe deformities. The symptoms of PD are frequently accompanied by varying degrees of ED. Pain may or may not be correlated with the development of penile curvature. The prevalence of PD is often underestimated. A survey of 152 primary care physicians (PCPs) and 98 urologists found that both urologists and, to a greater extent, PCPs underestimate the prevalence of PD and the rate of ED in patients with PD and overestimate the rate of spontaneous resolution. In addition, 44% of PCPs and 17% of urologists reported that they do not examine the penis as part of a routine physical, which the authors note is a missed opportunity to allow a patient to bring up any concerns he might have. PD represents localized aberration of the wound-healing process. The prerequisites to the development of PD in susceptible individuals include intercourse-related penile trauma, blunt penile trauma, bleeding within the tunica albuginea of the corpus cavernosum, clustering of fibrin and the inflammatory cells, and overexpression of cytokines and growth factors, which stimulate the production of more matrix proteins and inhibit the action of metalloproteinases. Growth factors such as TGF-beta are able to recruit more inflammatory cells and thus form a vicious cycle. The outcome is a prolonged inflammatory process in a matrix of excessive but disorganized elastic and collagen fibers resulting in focal loss of elasticity of the tunica albuginea. Furthermore, PD has been commonly associated with ED. The four factors that contribute to ED are severe penile deformity preventing intercourse, a flail penis, psychological distress or performance anxiety, and impaired penile vascular function. It has also been proposed that the reduced compliance of the tunica albuginea of the plaque prevents normal compression of these veins during erection. Patients usually present in either the early or late phase of the disease process. A patient in the early phase typically presents with a nodule or plaque, painful erection, and/or penile deformity during erection. In the late phase a patient presents with a harder plaque, stable penile deformity during erection and ED. The diagnosis is easily made with detailed medical and sexual history and physical examination. A detailed psychosexual history should also be obtained that includes penile rigidity during erection, shortening, induration, hourglass constriction, or pain with or without erection, and psychological impact of the disease. Physical examination usually detects a well-defined penile plaque or an area of palpable induration located on the dorsal surface of the penis with a corresponding dorsal penile deformity. Lateral and ventral plaques are less common but result in more coital difficulty. Penile pain may be present with erection or during sexual intercourse. Spontaneous improvement in pain usually occurs within 6 months as the inflammation settles. Penile ultrasonography is useful in identifying the number and site of the plaques and calcification. The treatment of PD is usually conservative at the outset. Reassurance is all that is necessary in patients with a slight curvature (less than 10 degrees) and no ED. If treatment is required, patients can be managed medically or surgically. The efficacy of medical management of PD is difficult to determine because, in the past, few studies were properly done. Treatment is aimed at correction of the penile deformity. Medical therapies include systemic agents and local or intralesional injections. Oral systemic agents include potassium aminobenzoate, tamoxifen, acetyl-L-carnitine, colchicine, and vitamin E. In an uncontrolled study, colchicine was shown to decrease plaque size and improve penile curvature in approximately 50% of the 24 patients treated. The main side effect of the medication is gastrointestinal upset and diarrhea. Potassium aminobenzoate has been used extensively for PD, although its mechanism of action is not well understood. An extensive review of this therapy found it to be successful in 57% of cases. The most frequent side effect is gastrointestinal upset. Several reports, although small, uncontrolled, and short-term, suggest mild to moderate benefits of using tamoxifen. It is thought that tamoxifen facilitates the release of TGF-beta from fibroblasts. It has minimal side effects, including gastrointestinal distress and alopecia. Historically, vitamin E has been used to treat PD. However, a recent study compared the effects of vitamin E treatment and the natural progression of PD. They noted no significant differences between the two groups with respect to pain, bend, intercourse, and overall perception of disease progression. Many intralesional therapies for PD have been used, including injection of calcium channel blockers, steroids, and interferons. Purified clostridial collagenase is currently under study. The use of verapamil as an intralesional agent has been studied. A prospective study showed a decrease in curvature in about 80% of the patients. The use of collagenase as an intralesional agent has been subjected to a number of double-blind placebo-controlled protocols, and, in all, good efficacy has been seen. However, collagenase is currently available only as part of clinical trials. Other mechanical devices such as the vacuum or devices for penile traction have not been adequately studied. Intralesional steroids have many local side effects, including local tissue atrophy and skin thinning with little improvement in well-established curvature. Surgical treatment is an option for all patients including those with severe curvature or narrowing that interferes with sexual intercourse. Penile surgery should be delayed until the disease has stabilized, typically 6 to 18 months after onset. Detailed evaluation of penile vascular and erectile function (EF) is highly recommended before surgical intervention, via a penile ultrasound with an injection of a vasoactive agent to induce an erection. Patients need thorough counseling with respect to the expected outcomes and possible side effects of surgery. Historically, penile implants were the only therapeutic option for patients with severe curvature with or without preserved EF. Surgical reconstruction is generally divided into three categories: tunical shortening procedures, tunical lengthening procedures, and prosthetic procedures. Tunical shortening procedures are performed on the convex side of the penis opposite the penile plaque. Shortening procedures are most appropriate for patients with good EF, adequate penile length, and no hourglass or narrowing type of deformity. The technique of elliptical excision of the tunica to treat PD was introduced by Pryor and Fitzpatrick in 1979. In a review of 359 men operated on between 1977 and 1992, 82% regained the ability to have intercourse. Complications reported after the Nesbit procedure include penile shortening, ED, penile hematoma, penile narrowing or indentation, urethral injury, herniation, suture granuloma, glans numbness, and phimosis. Other modifications include the Yachia procedure. Instead of removing an ellipse of tunica, a long longitudinal or multiple smaller longitudinal incisions are made in the tunica albuginea and are then closed horizontally in a Heineke-Mikulietz fashion to correct the angle of penile curvature. Tunical lengthening procedures involve lengthening the curved, shortened side to straighten the penis; this is performed by incising or excising the plaque on the short side of the penis and placing a graft to cover the defect. Lengthening procedures are indicated in patients with severe penile curvature or narrowing or hourglass deformities. Tunical grafting met with limited success until the introduction of dermal grafts. Many autologous grafts (temporalis fascia, dura mater, tunica vaginalis, and saphenous vein), cadaveric tissue (dermis, fascia, pericardium, or porcine small intestine submucosa), and synthetic materials (polyester and polytetrafluoroethylene) have subsequently been used with varying results. Excision of the plaque has been the standard approach. However, the pathological process of PD often extends far beyond the plaque, and removing a large area of tunica albuginea may impair EF. Successful straightening was accomplished in 75% to 95% of the patients, with 5% to 13% of potent men complaining of a decrease in EF after surgery. Thus some surgeons have moved away from plaque excision and have moved to plaque incision with placement of a graft. The use of a penile prosthesis in patients with PD is reserved for those with severe curvature with or without preserved ED that has not responded to medical management. Excellent results have been reported in the literature. Adjunct procedures during prosthesis placement include incising or excising the plaque during prosthesis placement. In most patients with mild to moderate curvature, insertion of a penile prosthesis tends to straighten the penis, and no additional procedures are necessary. Complex interactions between physiologic, neuroendocrine, and vascular mechanisms and psychogenic interplay produce penile erection. Sexual stimulation triggers a cascade of events. In essence, erections are neurovascular phenomena combining neurotransmission and vascular biologic responses. A release of neurotransmitters results in smooth-muscle relaxation in both penile erectile tissue and the penile arterial walls. This transforms the penile vasculature and erectile tissues from a contracted, minimally perfused state to a relaxed, blood engorged state. 1. Functional anatomy of the penis a. Corporal bodies EJD is subdivided into premature or rapid ejaculation, delayed ejaculation, anejaculation, or retrograde ejaculation. Retrograde ejaculation occurs when the bladder neck does not close as the seminal fluid or ejaculate reaches the prostatic urethra. Thus this fluid enters the bladder instead of expulsion outside the body. Delayed ejaculation can be due to a dysfunctional nervous system, such as seen in diabetes or other neurological conditions that dampen the nervous impulses, or may be due to psychogenic causes. The same holds true of anejaculation. Psychologically, one theory is that the male is “punishing” his partner by not ejaculating. This condition can be so severe, that electroejaculation or sperm aspiration may be required to procure sperm for fertility. Rapid ejaculation, as noted in the introduction, is enigmatic. Rapid ejaculation is the rule in the animal kingdom as the animal wishes to mate quickly to avoid predators. Humans have different motivational issues, so that the definition of rapid is not as clear. The historical definition is ejaculation outside the vagina or just upon entrance, within 1 to 2 minutes of an erection. The newly proposed definition is: Lifelong PE in heterosexual men is ejaculation occurring within approximately 1 minute of vaginal penetration on 75% of occasions for at least 6 months. Acquired PE is a condition in which the man suddenly ejaculates rapidly after some incident or event, whereas he was ejaculating “normally” prior to this event. Normal ejaculatory times or latencies were described by Waldinger et al. The duration of ejaculation as measured by intravaginal ejaculation latency time (IELT) may give rise to subjective complaints of PE and is usually determined by self-assessment or by stopwatch. Waldinger’s group investigated the IELT distribution in the general male population and the accuracy of IELT assessment by using a blinded timer device instead of a stopwatch, thereby minimizing possible interference with the spontaneous and natural way of having intercourse. The IELT was measured with a timer device during 4 weeks in a nonselected sample of 474 men from the Netherlands, Spain, United Kingdom, Turkey, and the United States. Questionnaires were administered before and after the 4-week IELT assessments. The data revealed that the IELT had a positively skewed distribution, with a geometric mean of 5.7 minutes and a median of 6 minutes (range: 0.1 to 52.1 minutes). Men from Turkey had the shortest median IELT (4.4 minutes). Men from the United Kingdom had the longest IELT (10 minutes). Circumcision and condom use had no significant impact on the median IELT. Subjects who were discontent with their latency time had slightly lower median IELT values of 5.2 minutes than the median of the population. These help provide guidance on how to describe “normal” ejaculatory periods. PE is defined as noted above. Delayed ejaculation and anejaculation caused by psychogenic issues are treated with psychotherapy. Retrograde ejaculation can be treated with oral sympathomimetics taken within a few hours of sexual activity. PE is best treated with a combination of psychotherapy and drug therapy. There are topical agents to numb the glans penis and oral pills to delay ejaculation such as SSRIs. Other less attractive therapies include penile dorsal nerve transection and percutaneous dorsal nerve ablation. ED is defined as the inability of the male to achieve an erect penis as part of the overall multifaceted process of male sexual function. This definition is not limited to intercourse and gives equal importance to other aspects of male sexual behavior.
Male Sexual Dysfunction
Introduction
Peyronie disease
Burden of disease
Pathogenesis
Clinical presentation and evaluation
Treatment
Physiology of sexual function
Erection
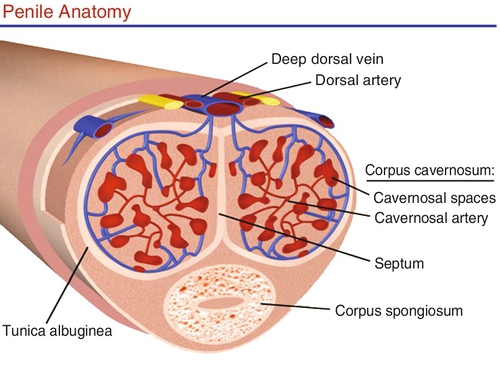
The penis is composed of three cylindrical structures: the paired corpora cavernosa and the corpus spongiosum. The corpora cavernosa comprise two spongy, paired cylinders contained in the thick envelope of the tunica albuginea, which is covered by a loose subcutaneous layer underlying the skin. The tunica affords great flexibility, rigidity, and tissue strength to the penis. Between these layers of tunica run the emissary veins, which are compressed during erection, ensuring high-pressure rigidity. In the ventral groove of the tunica albuginea (between the 5 o’clock and 7 o’clock position), the outer layer is absent, leaving this area most vulnerable to penile prostheses extrusion. Each corpora cavernosum houses a network of endothelial-lined sinusoids within a trabecula of smooth muscle. An incomplete septum between them allows passage of blood from one side to the other. The terminal cavernous nerves and helicine arteries are intimately associated with the smooth muscle. In the flaccid state, the blood slowly diffuses from the central to the peripheral sinusoids. During erection, the rapid entry of arterial blood to both the central and the peripheral sinusoids expand and fill the cavernosa, producing an increase in pressure and a firm erection (Figure 20-e2).
The corpus spongiosum covers the urethra and expands distally to form the glans penis. The spongiosum is covered with only one layer of tunica albuginea, allowing low pressure during erection. The glans penis has a high concentration of nerve receptors to provide sensory input to facilitate erection and enhance pleasure.
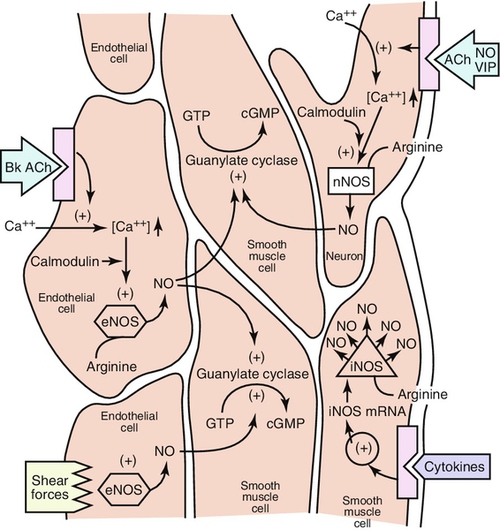
In corporal cavernosal tissue, nitric oxide (NO) is accepted as the principal neurotransmitter responsible for erectile response (Figure 20-e3). With sexual arousal, NO synthase converts L-arginine and oxygen to NO. NO is also released from nonadrenergic-noncholinergic nerves and endothelial cells to cause an increase in the production of cyclic guanosine monophosphate (cGMP), a second messenger, which activates protein kinase G. NO enters into the target cell and binds to guanylate cyclase, launching a signaling pathway which ultimately produces penile cavernosal smooth-muscle relaxation via formation of cGMP. This leads to the opening of potassium channels and the closing of calcium channels, which ultimately lead to a drop in cytosolic-free calcium. This drop in calcium is the direct cause of arterial and cavernous smooth-muscle relaxation, culminating in increased penile blood flow. Thus the cyclic nucleotide signaling pathway mediates the smooth-muscle relaxing effects of NO necessary for normal EF. Down-regulation of this pathway is thought to be central to the pathophysiology of many forms of ED.
Cyclic nucleotide levels are determined by both synthesis, through the activities of guanylate cyclase on GTP, and enzymatic degradation, through the activity of phosphodiesterases (PDEs). Penile detumescence is in part the result of an increase in phosphodiesterase inhibitor type 5 (PDE-5) activity, which breaks down cGMP. The smooth muscle regains its contractile tone when cGMP is degraded by PDE-5. Competitive inhibition of the action of PDE-5 with drugs such as PDE-5 inhibitors, sildenafil, vardenafil, and tadalafil enhances erection. These drugs prevent the degradation of cGMP, allowing cGMP to act longer and produce an erection of better quality and longer duration.

Ejaculatory dysfunction
Treatment of ejaculatory dysfunction
Pathophysiology of erectile dysfunction
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


