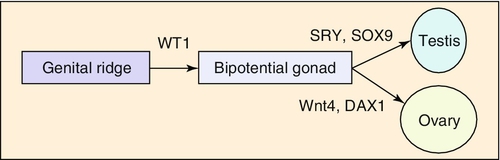
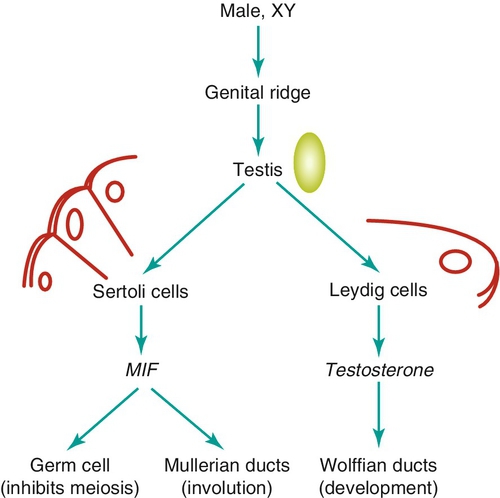
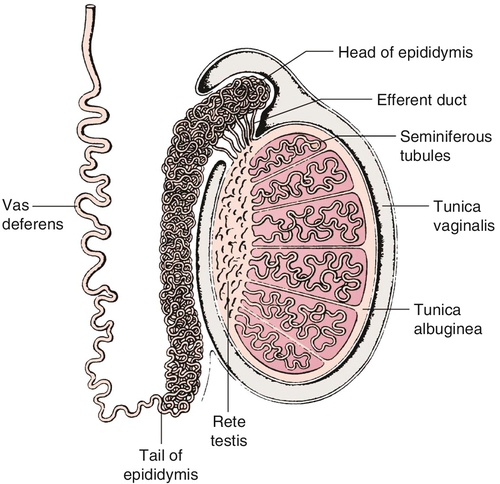
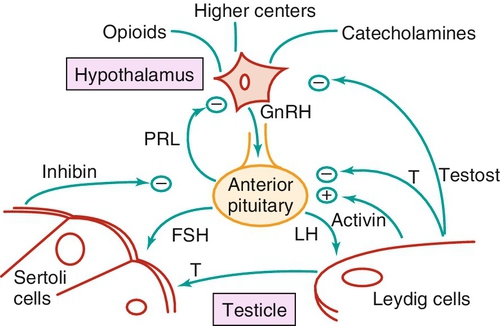
Chapter 21 2. Upon evaluation, a male factor alone may be found in approximately 30% of couples, and both a male and female factor in an additional 20%. Thus a male factor is involved in the infertility problem in approximately 50% of cases. 2. The indifferent gonad forms from a thickening in the urogenital ridge near the mesonephros; germ cells migrate from the yolk sac to populate the urogenital ridge. These primordial germ cells are very closely related to embryonic stem cells. 3. Sexual differentiation of the embryo stems from the presence or absence of testis determining factor (TDF or SRY) located on the Y chromosome. This determines gonadal sex and forms the basis for gender-specific phenotypic development (Figure 21-e1). Early embryonic genes that can modulate the influence of SRY include WNT-4 and DAX1, both of which favor female gonadal development. 4. Induced to form a testis, the gonad develops clustered cords of germ cells that converge to form the rete testis at the hilum of the testis. These testis stem cells serve as the renewing source of germ cells for spermatogenesis throughout life. To further underscore the relatedness of stem cells, human testicular stem cells can be “coaxed” into developing into cells virtually identical to embryonic stem cells. 5. During the eighth week of gestation, testosterone is made by differentiating Leydig cells. Hormone production declines after the twelfth week during which external genital development occurs (Figure 21-e2). 6. The mesonephric duct forms the ureter in both sexes and regionally specializes to form the vas deferens and epididymis in the male, joining with the testis at the ductuli efferentes testis. 7. The mullerian duct develops into fallopian tubes, the uterus, and the upper portion of the vagina in the female; in the male this development is inhibited by a mullerian-inhibiting substance (mullerian-inhibiting factor; MIS, MIF) produced by the primitive testis. Except for the appendix testis and prostatic utricle, regression is otherwise complete (see Figure 21-e2). 8. Late in gestation, the testis descends caudally along the posterior abdominal wall as a result of differential growth and through shortening of the gubernaculum testis within the scrotum under endocrine control. Descent into the scrotum is usually completed by birth, although it can still occur during the first year of life. 2. From the standpoint of infertility, any consideration of anatomy must also include the hypothalamic-pituitary-gonadal axis. a. Extrahypothalamic central nervous system (CNS) b. Hypothalamus c. Pituitary d. Testes e. Steroid sensitive organs 2. Functions a. Normal male sexual development b. Maintenance of secondary sexual characteristics c. Male sexual behavior d. Sperm production and maturation 2. In humans, the effects of stress of both a physical and/or emotional nature are probably mediated through this system, but the mechanisms are unknown. 2. The hypothalamus is responsible for production of gonadotropin-releasing hormone (GnRH) which is the primary releasing substance involved in male sexual function. 2. Both LH and FSH are glycopeptides with two molecular chains. They share a common alpha chain; specificity is determined by a unique beta chain. 3. LH and FSH are secreted episodically. LH is rapidly metabolized, causing wide swings in blood concentrations when determined by radioimmunoassay techniques. Occasionally, more accurate LH levels are needed; this is done with pooled blood samples. FSH is more slowly metabolized, resulting in a more constant level within the bloodstream. 4. The testes are the primary target for LH and FSH. No other target organs for these hormones have been found. b. The interstitium between the seminiferous tubules contains blood vessels, lymphatics, and Leydig cells. 2. Seminiferous tubule structural organization b. Sertoli cells have membrane receptors that bind FSH, resulting in increased intracellular cAMP and subsequent cytoskeletal reorganization for protein synthesis. c. The primary secretion products from Sertoli cells include MIS in the fetus, androgen-binding protein (ABP), transferrin, and inhibin (a nonsteroidal glycoprotein) in the adult. d. Sertoli cells regulate the tubule microenvironment. They govern fluid secretions into the lumen of the seminiferous tubules and partake in phagocytosis, steroid metabolism (in part), sperm production, and sperm movement through the tubule. e. Sertoli cells are also mainly responsible for the blood-testis barrier. This anatomic barrier is surrounded by myoid cells and consists of the basement membrane and Sertoli cell tight junctions. In addition, a selective physiologic barrier is created by active transport processes across Sertoli cells. Further compartmentalization is established as Sertoli cells divide, developing germ cells into two areas, the basal compartment for immature germ cells and stem cells and the adluminal compartment for germ cells undergoing differentiation and maturation. This complex blood-testis barrier provides an immunologically privileged site for mature spermatozoa because these haploid cells harbor unique and specific antigens that are not otherwise recognized as “self” by the body’s immune system. 3. Seminiferous tubules: spermatogenesis a. LH, FSH, and testosterone are all required for normal spermatogenesis. b. Sertoli cells, lining 250 meters of seminiferous tubules in the average testis, regulate the complex process of spermatogenesis. c. A variety of germ cell types exist, including spermatogonia, primary spermatocytes, secondary spermatocytes, spermatids, and spermatazoa. Fourteen germinal cell subtypes are recognized histologically and are associated with six distinct stages of spermatogenesis. Certain early-stage spermatogonia are actually adult testis stem cells and are the source of the constantly self-renewing germ-cell lineage. Spermatids and spermatozoa have a haploid complement of chromosomes. d. The process of spermatogenesis takes about 60 days to complete. The average daily output is 125 million spermatozoa, which declines with age. A normal man makes 1200 sperm for every heartbeat. e. Spermiogenesis is the maturation process of a spermatid to a spermatozoan. This includes nuclear condensation and a programmed repackaging of DNA from histones to protamines, acrosome formation, residual body separation from the sperm, and tail formation. It is one of the most complex series of morphological changes undertaken by any mammalian cell. 4. Interstitium: Leydig cells b. LH stimulation results in the conversion of cholesterol to testosterone in a steroidogenic pathway. c. Testosterone diffuses into the plasma (endocrine function) or into the seminiferous tubule lumen (paracrine function). In the plasma, testosterone is bound (98%) to sex hormone-binding globulin (SHBG) or albumin. Within the seminiferous tubules, testosterone is bound to ABP. d. Depending on the target tissue, testosterone may be active itself or may be reduced to dihydrotestosterone (DHT) by the enzyme 5-alpha reductase. e. Testosterone is responsible in part for sexual differentiation, spermatogenesis, gonadotropin regulation, sexual maturation, and behavior. 2. LH regulation b. Testosterone therefore regulates its own production by acting on the pituitary and hypothalamus. This has implications for clinical care: Exogenous testosterone supplements will suppress both endogenous testosterone (through decreased LH) and sperm production (through decreased FSH). c. Estradiol is produced within the testicle and the liver upon conversion from testosterone (5-alpha reductase). It is found in smaller amounts within the blood stream (testosterone:estrogen ratio is typically 10:1) but is potent in action. The site of regulation is also at the level of the pituitary and hypothalamus. 3. FSH regulation b. In man, Sertoli cells produce inhibin, a two-subunit hormone in the transforming growth-factor family, which has an inhibitory effect on pituitary FSH output. In contrast, activin, a glycoprotein formed as a homodimer of either inhibin chain, has a stimulatory effect on pituitary FSH. Neither inhibin nor activin affects pituitary LH release. 4. A variety of short feedback loops and other modulating substances more finely tune this system. 2. The movement of the spermatozoa from the testis to the epididymis is controlled by four factors: a. Fluid pressure generated within the seminiferous tubule b. Myoepithelial contractions of the seminiferous tubules c. Contraction of the tunica albuginea of the testis d. Cilia within and contraction of the wall of the efferent ductules 3. The spermatozoa enter the epididymis in an immature state. b. Approximately 700 million sperm are stored within the epididymides and vasa deferentia. Approximately 60% of these are stored within the tail (cauda) of the epididymides. Sperm become progressively more motile as they traverse the epididymal tubules, knowledge of which is important in harvesting sperm from the epididymis for in vitro fertilization (IVF) with intracytoplasmic sperm injection (ICSI). c. At the time of emission, regular coordinated contractions of the tails of the epididymides and the vasa deferentia occur, mediated by the sympathetic nervous system, propelling sperm into the prostatic urethra. During ejaculation, somatic nervous system-stimulated rhythmic contractions of periurethral and pelvic floor muscles propel the sperm through the urethra in the presence of a closed bladder-neck closure mediated by the sympathetic nervous system. 2. Sperm maturation b. A variety of sperm membrane changes in permeability and antigenicity also occurs. c. Motility and fertilizing capacity are gained during transport through the epididymis. d. The final process of maturation, sperm capacitation, takes place after the sperm have been ejaculated and come in contact with the female reproductive tract. Fertilizing capacity lasts approximately 48 hours within the external female genitalia, an important finding for counseling patients on the optimum frequency of sexual intercourse around the time of ovulation. 2. Prostatic fluid b. Specific prostate products include liquefaction factors such as prostate-specific antigen (PSA), zinc, citric acid, acid, phosphatase, and spermine. The latter substance, when oxidized to aldehydes, produces the characteristic odor of semen. c. PSA, a 33-kd molecular weight serum protease in the family of glandular kallikreins, serves to liquefy the coagulum of human semen 5 to 20 minutes following ejaculation. 3. Seminal vesicle fluid b. Specific substances secreted by the seminal vesicles include coagulation factors, prostaglandins, and fructose. Fructose is measured on a semen analysis to investigate the diagnosis of ejaculatory duct obstruction. b. Contraceptive methods and duration used c. Length of time trying to conceive d. Number of pregnancies, including miscarriages and therapeutic abortions, which indicate the potential to conceive 2. Previous marital history and relationships b. The duration and number of pregnancies conceived by the wife or partner should also be determined. c. Previous marriages are common. Potential fertility problems may be suspected if one partner has previously attempted to conceive without success. However, remember that the ability to conceive is a phenomenon involving both partners, and therefore is ultimately determined by the current couple. 3. Sexual history b. Libido, potency, and sexual technique: A normal desire and ability to have intercourse is critical, and problems in these areas are often overlooked by clinicians. Situational erectile dysfunction (ED) caused by stress is common among couples trying to conceive and is easily treated with phosphodiesterase inhibitors. c. Ejaculation: This needs to occur deep within the vagina. Severe problems with premature ejaculation, chordee, or severe hypospadias may prevent proper deposition of sperm. d. Dyspareunia and the use of lubrication: Impaired natural vaginal lubrication can result in painful intercourse for either partner. Most lubricants are spermicidal. The use of vegetable oil-based substances is safe for sperm. e. Understanding of the ovulatory cycle: It is important that the couple understand when ovulation occurs and have timed intercourse regularly during this time. There is evidence that “front loading” sexual intercourse, having more before rather than after ovulation, might improve pregnancy rates. 4. Genitourinary history b. Sexual development and onset of puberty: Delayed puberty can indicate syndromes (e.g., Kallmann) or chromosomal issues (e.g., XXY). c. Infections: Venereal, nonvenereal, mumps (at the time of puberty or later), recent febrile illness, or other infectious problems that directly involve the genitalia or urogenital duct system may be associated with a significant scarring and subsequent fertility problems. Viral infections and other febrile illnesses not specifically involving the genitalia may also temporarily lower sperm counts.
Male Fertility and Sterility
Background
The problem
Reproductive anatomy and physiology
Embryology
Gross anatomy (Figure 21-e3)
Reproductive hormonal axis (Figure 21-e4)
Extrahypothalamic central nervous system
Hypothalamus: gonadotropin-releasing hormone
Pituitary: luteinizing hormone and follicle-stimulating hormone
The testes
Feedback mechanisms (see Figure 21-e4)
Testicular transport
Epididymal functions
Semen composition
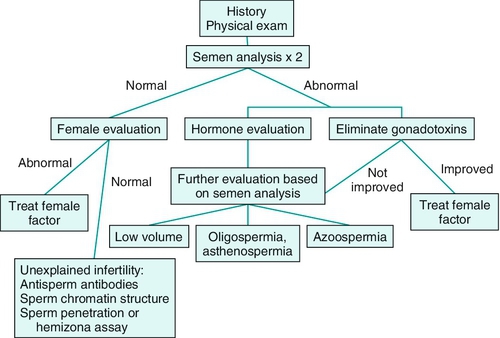
Clinical evaluation of the subfertile male (Figure 21-1)
Fertility history
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree



