Lymphomas of the Gastrointestinal Tract
Andrew D. Zelenetz
The non-Hodgkin lymphomas (NHLs) are a broad spectrum of lymphoid malignancies arising from B cells, T cells, and natural killer (NK) cells, as shown in Table 49.1 (1). Gastrointestinal tract (GIT) involvement is seen either as part of a systemic disease (e.g., diffuse large B cell lymphoma [DLBCL], mantle cell lymphoma [MCL], follicular lymphoma [FL], or Burkitt lymphoma [BL]) or as an entity with primary GIT involvement (e.g., mucosa-associated lymphoid tissue [MALT] lymphoma of the stomach or bowel, immunoproliferative small bowel disease [IPSID], or enteropathy-associated T-cell lymphoma [EATL]). Over the past 10 to 15 years there has been a dramatic change in the management of the systematic lymphoma with common GIT involvement particularly involving the stomach with a move away from surgical management. Randomized studies in DLBCL and MALT involving the stomach have demonstrated that gastrectomy (either total or subtotal) does not improve outcome. This chapter will review the biology and management of those lymphomas that primarily involve the GIT and will discuss management issues associated with systemic lymphomas with frequent GIT involvement.
Gastric Malt Lymphoma
MALT lymphoma is the prototype of a localized indolent B cell lymphoma. MALT lymphomas can involve a wide variety of mucosal sites including the stomach, small bowel, and colon; however, involvement is often organ confined. MALT lymphomas can arise at a variety of sites outside the GIT including salivary glands, respiratory tract, ocular adnexa, thyroid, liver, breast, or genitourinary tract; however, discussion of the non-GIT MALT lymphoma is beyond the scope of this chapter.
Pathology
MALT lymphomas arise from sites normally devoid of lymphoid tissue that become colonized with lymphoid cells as a consequence of chronic inflammation, infection, or autoimmune reactions (2). In gastric MALT lymphoma, the tumors are frequently multifocal, and the gross appearance can range from that of chronic gastritis to the presence of distinct masses. The histologic features of MALT lymphoma have been well described (3,4,5). Morphologically, the tumor cells may have the appearance of centrocytes or small lymphoid cells, or they may be monocytoid. The presence of somatic hypermutation in the rearranged immunoglobulin heavy chain (IgH) gene indicates an origin from a postgerminal center B cell (6). The pattern of somatic mutation in gastric MALT lymphoma suggests that antigen stimulation is involved in tumor development (6,7). Specimens need to be examined carefully for transformed large cells as transformation to aggressive lymphoma can be seen at presentation; furthermore, the presence of >10% of large cells in the biopsy is associated with shorter survival (8). The type of biopsy specimen, endoscopic biopsy versus gastrectomy, has been reported to influence histologic categorization (8). Biopsies obtained with endoscopy resulted in significantly fewer diagnoses of a high-grade large cell component compared to gastrectomy specimens. An important histologic finding is the presence of lymphoepithelial lesions (LELs) formed by the invasion of individual glands by aggregates of lymphoma cells (2). The presence of LELs is associated with longer survival compared to tumors having >10% high-grade cells (8). The immunophenotype of MALT lymphoma is not highly distinctive and is the same as marginal zone B cells: CD20+, CD21+, CD35+, IgM+, IgD–, CD5–, cyclin D1– (9); given the similarity to the marginal zone immunophenotype, the MALT lymphomas are categorized as a subset of the marginal zone lymphoma in the World Health Organization (WHO) Lymphoma Classification (1).
The evaluation of post-treatment biopsies can be challenging; chronic lymphoid infiltration is commonly observed even when tumor has been eradicated. Several sets of criteria have been proposed to aid in post-treatment evaluation (Tables 49.3,49.4,49.5). A histologic score has been published to aid in the diagnosis of gastric MALT lymphoma and to distinguish active disease from post-treatment effect (10); the widespread application of this scoring system is limited by difficulty in applying the criteria. Two additional sets of criteria have been proposed (11,12). The Groupe d’Etude des Lymphomas Agressifs (GELA) criteria in Table 49.5 have proven to be reproducible with a high degree of inter-observer concordance. Molecular assays for residual disease with either the clonotypic polymerase chain reaction (PCR) or PCR for the IgH gene rearrangement (IgH PCR) assays are not useful in the identification of clinically significant disease; in both of these assays a significant proportion of the population demonstrate persistence of clonal B cells despite the absence of histologic or clinical evidence of disease (13,14).
Association With Helicobacter pylori
Gastric MALT lymphoma is strongly associated with H. pylori infection, and infection is felt to have an etiologic role in infected patients though chronic antigen stimulation. In vitro studies have demonstrated that infiltrating T cells respond to H. pylori in an antigen-specific manner. These H. pylori-activated T cells provide a stimulus for B-cell proliferation. As the tumor evolves, the B-cell lymphoma proliferation becomes independent of T-cell help (2,15). Eradication of the H. pylori infection with antibiotic treatment can result in clinical regression of
clinical stage IE gastric MALT lymphoma in approximately 70% of patients. Large, deeply invasive tumors and those that have undergone high-grade transformation typically do not respond to antibiotic therapy (2,10,16). Despite the numerous studies demonstrating that clinical remissions can be induced by antibiotics in the majority of localized cases, molecular testing reveals persistence of disease in at least 50% of cases (17). Late relapses occur, which are sometimes self-limited, and careful clinical monitoring is essential. Some patients fail to respond to or recur following antibiotic therapy; this can be seen particularly in patients with specific chromosomal translocations (Fig. 49.1). The most common of these translocations is the t(11;18) translocation resulting in a unique fusion protein involving API2 and MALT1 (18,19,20). Patients with antibiotic-resistant disease can be effectively managed with involved field radiation therapy (IFRT), with excellent long-term disease control (21). Molecular studies using tumor-specific clonotypic PCR have demonstrated that, even after radiation therapy, tumor B cells persist in blind gastric biopsies despite absence of clinical relapse; these findings suggest that the radiation altered the microenvironment in a manner that no longer favored lymphoma growth (14).
clinical stage IE gastric MALT lymphoma in approximately 70% of patients. Large, deeply invasive tumors and those that have undergone high-grade transformation typically do not respond to antibiotic therapy (2,10,16). Despite the numerous studies demonstrating that clinical remissions can be induced by antibiotics in the majority of localized cases, molecular testing reveals persistence of disease in at least 50% of cases (17). Late relapses occur, which are sometimes self-limited, and careful clinical monitoring is essential. Some patients fail to respond to or recur following antibiotic therapy; this can be seen particularly in patients with specific chromosomal translocations (Fig. 49.1). The most common of these translocations is the t(11;18) translocation resulting in a unique fusion protein involving API2 and MALT1 (18,19,20). Patients with antibiotic-resistant disease can be effectively managed with involved field radiation therapy (IFRT), with excellent long-term disease control (21). Molecular studies using tumor-specific clonotypic PCR have demonstrated that, even after radiation therapy, tumor B cells persist in blind gastric biopsies despite absence of clinical relapse; these findings suggest that the radiation altered the microenvironment in a manner that no longer favored lymphoma growth (14).
Table 49.1 WHO classification of the non-Hodgkin’s lymphomas | ||
|---|---|---|
|
Table 49.2 Staging studies in MALT lymphoma | |||
|---|---|---|---|
|
Table 49.3 Histologic score for diagnosis and post-treatment evaluation of gastric MALT lymphoma as proposed by Wotherspoon et al. | ||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| ||||||||||||||||
Table 49.4 Criteria for diagnosis of gastric MALT lymphoma and post-treatment evaluation as proposed by Neubauer et al. | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| ||||||||||
Table 49.5 GELA histological grading system for post-treatment evaluation of gastric MALT lymphoma | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| ||||||||||||
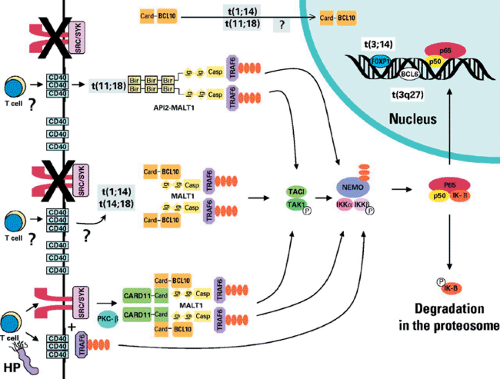 FIGURE 49.1. Molecular pathways in gastric mucosa-associated lymphoid tissue (MALT) lymphoma. T cells specific for Helicobacter pylori signal the B cell via CD40 and the immunoglobulin receptor (IGR). BCL10 interacts with CARD11 and MALT1-activating tumor necrosis factor (TNF) receptor activating factor 6 (TRAF6) promoting phosphorylation of IκB and thereby activation of NF-κB. The needs for IGR or CD40 activation is lost (or significantly diminished) with the t(1;14) translocation resulting in BCL10 overexpression or t(14;18) resulting in MALT1 overexpression. Similarly, the novel fusion protein API2-MALT1 produced as a consequence of the t(11;18) translocation bypasses the need for signaling via the IGR and CD40 and can interact with TRAF6 to activate NF-κB. Reprinted with permission from Farinha P, Gascoyne RD. Molecular pathogenesis of mucosa-associated lymphoid tissue lymphoma. J Clin Oncol. 2005;23:6370–6378 (See also color Figure 49.1). |
Clinical Evaluation of MALT Lymphoma
Gastric MALT lymphoma most often presents as organ-confined disease, although it can frequently be multifocal. Molecular evaluation of gastrectomy specimens has revealed that disease is distributed throughout the gastric mucosa (22). This finding explains the high risk of late recurrence in patients treated with partial gastrectomy. However, retrospective studies of patients with gastric MALT lymphoma have reported that up to one-third of patients with gastric MALT lymphoma present with disseminated disease at diagnosis (23,24). Therefore, careful staging to exclude dissemination to other MALT sites is important. A prospective study of extensive staging in MALT lymphoma has been performed (25). The staging evaluation included ophthalmologic examination; otolaryngologic investigation; gastroscopy with multiple biopsies; endosonography of the upper GIT; enteroclysis; colonoscopy; computed tomography of the chest, abdomen, and pelvis; and bone marrow biopsy. Eight of 35 patients (23%) had simultaneous involvement of two MALT sites; among 11 cases of low-grade gastric MALT lymphoma, two cases with extragastric involvement were identified (one case of colon and one case of lung involvement). In a follow up series, the same authors reported 15 of 61 (25%) of gastric MALT lymphomas had multiorgan involvement (26). Lung and colon were the most common second sites in these patients with gastric MALT lymphoma. Among the nongastric MALT lymphomas, the risk of multiorgan involvement was 46% (37 of 79), with 9 of the 37 cases involving the stomach as the second site. When multiorgan involvement is identified, it is impossible to definitively determine the initial site of involvement; rather, the initial site is based on the clinical presentation. However, in some cases of simultaneous gastric and intestinal disease, DNA sequencing of the rearranged IgH variable gene has demonstrated a common clonal origin for the gastric and intestinal disease; the pattern of somatic mutations
observed suggested that the primary disease arose in the stomach (7). Bone marrow involvement was uncommon, being identified in only 3 of 140 patients with MALT lymphoma; 2 patients were among the 61 with gastric MALT lymphoma. The presence of the t(11;18) translocation was significantly associated with the risk of dissemination among the patients with gastric MALT lymphoma; trisomy 18 was associated with dissemination of the nongastric MALT lymphomas. It is interesting that survival was not different for the patients with localized versus multifocal disease. The simultaneous presence of MALT lymphoma at multiple sites is also suggested by patients who have clonally identical relapse at distant sites after definitive local therapy; two reports of late recurrence of gastric lymphoma have been described with involvement of the lung, small bowel, and gall bladder (27,28). These data support the need for a thorough and careful staging evaluation of MALT lymphoma (Table 49.2).
observed suggested that the primary disease arose in the stomach (7). Bone marrow involvement was uncommon, being identified in only 3 of 140 patients with MALT lymphoma; 2 patients were among the 61 with gastric MALT lymphoma. The presence of the t(11;18) translocation was significantly associated with the risk of dissemination among the patients with gastric MALT lymphoma; trisomy 18 was associated with dissemination of the nongastric MALT lymphomas. It is interesting that survival was not different for the patients with localized versus multifocal disease. The simultaneous presence of MALT lymphoma at multiple sites is also suggested by patients who have clonally identical relapse at distant sites after definitive local therapy; two reports of late recurrence of gastric lymphoma have been described with involvement of the lung, small bowel, and gall bladder (27,28). These data support the need for a thorough and careful staging evaluation of MALT lymphoma (Table 49.2).
Staging and Endoscopic Ultrasound
In gastric MALT lymphoma, evaluation of the local extent of the disease is achieved by upper gastroscopy. However, endoscopic ultrasound (EUS) has demonstrated more extensive disease when compared to conventional gastroscopy. Using EUS, four patterns of involvement of the stomach have been recognized: superficial spreading; infiltrating; mass-forming; and mixed type (29,30). The superficial spreading and the infiltrating patterns were strongly associated with low-grade MALT lymphoma, and the mass-forming pattern was associated with high-grade MALT or DLBCL.
The Ann Arbor staging system (31,32) has been modified for primary gastric lymphoma (33). The modification shown in Table 49.6 includes subdividing (CS IE) into IE1 (involving the mucosa and/or submucosa) and IE2 (in which disease extends beyond the submucosa). CS IIE disease is subdivided into IIE1 (involving perigastric lymph nodes) and IIE2 (involving lymph nodes beyond region nodes). The modified Ann Arbor staging system cannot distinguish the superficial spreading from infiltrating forms of gastric lymphoma.
The Tumor–Node–Metastasis (TNM) classification for gastric cancer staging has been applied to the staging of gastric lymphoma (Table 49.7) (34,35). T1 disease describes mucosal and submucosal involvement and is further subdivided into T1m (involving the mucosa only), T1sm (with extension to the submucosa), T2 (involving the muscularis propria), and T3 disease invading into the serosal layer. Regional nodal disease is designated N1, and the absence of regional nodes N0. Conventional radial EUS assesses T stage with a precision and sensitivity of 85% to 90% and N stage with a sensitivity of 40% to 90%. Miniprobes have been compared to conventional radial EUS probes and have been found to have similar determination of T stage and N stage (T1 53% vs 60%, T2 33% vs 20%, N1 53% vs 60% for miniprobe vs conventional probe) (36).
Table 49.6 Modified Ann Arbor staging system for primary gastric lymphoma | ||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| ||||||||||||||||||||
Table 49.7 TNM staging of gastric cancer | ||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| ||||||||||||||||||||||||||||
Unfortunately, the utility of EUS is limited somewhat by inter-observer variability (37). A multicenter study was conducted in which black and white thermal images were assessed by other observers to determine the T and N stage. At diagnosis there was only moderate inter-observer agreement in the designation of T1m disease and only fair agreement for patients with T1sm and T2 disease. At diagnosis there was substantial inter-observer agreement in the designation of N stage. High degree of inter-observer variability can potentially have important clinical consequences, as T stage is associated with response to therapy.
Diagnosis of H. pylori Infection
H. pylori is a ubiquitous organism colonizing half of the global human population (38). However, this organism has also been strongly associated with a number of disease states including gastritis, gastric and duodenal ulcers, gastric adenocarcinoma, and gastric MALT lymphoma. Eradication of the H. pylori infection can result in clinical remission of MALT lymphoma (see response of gastric malt lymphoma to eradication of H. pylori). This would suggest that an accurate diagnosis of H. pylori is imperative. However, some researchers have advocated that all patients with localized gastric MALT lymphoma be treated with an empiric course of therapy aimed at H. pylori eradication in part because of the difficulty in establishing a definitive diagnosis of infection. Empiric therapy is a reasonable strategy; however, the emergence of clarithromycin-resistant strains of H. pylori has complicated the interpretation of the results. Failure to respond may be the result of a failure to eradicate the infection or an inherent resistance to treatment because of molecular evolution of the clone (see Figure 49.1). In the first case, the appropriate treatment might be alternative
therapy directed at H. pylori; in the second, antitumor therapy with radiation or systemic treatment may be indicated. Thus, efforts should be undertaken to achieve an accurate diagnosis of H. pylori infection.
therapy directed at H. pylori; in the second, antitumor therapy with radiation or systemic treatment may be indicated. Thus, efforts should be undertaken to achieve an accurate diagnosis of H. pylori infection.
H. pylori can be detected by both noninvasive and invasive means. Invasive tests include endoscopy, with biopsy of the affected region with histopathologic examination of stained specimens to demonstrate the presence of the bacterium, rapid urease test, biopsy PCR, and culture of the bacterium. Upper GI endoscopy with biopsy and histologic examination has been the gold standard for the diagnosis of H. pylori infection, but this is costly and invasive. Fecal and saliva samples can be evaluated for H. pylori antigens using enzyme immunoassays or by PCR, although fecal testing has had greater reliability than saliva testing has had. Utility of PCR is limited by substances in the sample that inhibit the PCR; these substances can be addressed with pre-PCR treatment resulting in high specificity and sensitivity (39,40,41). The stool antigen test has also yielded high specificities (83% to 100%) and sensitivities (91% to 98%) in various regions of the world (41).
The gold standard among the noninvasive diagnostic methods has been the urea breath test (UBT), in which a patient is administered a dose of 13C-urea and the 13CO2 produced as a consequence of metabolism by the urease expressed by H. pylori is measured in the patient’s exhaled breath (42). Using biopsy as the gold standard, reported sensitivity and specificity of the UBT is 91.8% to 98.9% and 98.4% to 100%, respectively (43,44,45). However, the clinical utility of this test is limited by cost and the need for specialized equipment.
More recently, a number of studies have evaluated the accuracy of the fecal antigen tests with the UBT. A number of fecal antigen immunoassays are available. The sensitivity of these assays have been reported to be 73.4% to 100%; the specificities 92.5% to 100% (43,44,45,46). Most series have concluded that the UBT and fecal antigen tests yield similar results. Given the high diagnostic accuracy of these noninvasive tests, the choice of test between these two options should be made on the basis of cost and local facilities.
Clarithromycin is a macrolide that has become part of standard therapy for H. pylori (47). However, clarithromycin-resistant H. pylori has been emerging (48,49), with higher rates of resistance in the developing countries (25% to 50%) than in the United States (5% to 10%) and Europe (10%). The emergance of macrolide resistance led to the development of methods for the rapid detection of macrolide-resistant H. pylori in paraffin embedded or fresh samples (49,50,51). This method uses the fluorescence in situ hybridization (FISH) method to detect clarithromycin resistance due to mutations in the 2143 and 2144 positions of the 23S ribosomal RNA (rRNA) gene. This test can be used in populations at high risk for macrolide resistance prior to therapy to clarify the cause of treatment failure in patients with persistence of infection after standard therapy (51).
Table 49.8 Gastric MALT lymphoma response to Helicobacter pylori eradication | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
Response of Gastric MALT Lymphoma to Eradication of H. pylori
Numerous series have validated the original observation by Wotherspoon et al. (10) that histologic remission could be achieved by the eradication of H. pylori infection; however, these data are limited by relatively few patients and relatively short follow-up for a disease with a long natural history. Various regimens have been used for H. pylori eradication including dual and triple therapy. The most common regimens include a proton pump inhibitor(PPI) with amoxicillin 1 g orally twice daily and clarithromycin 500 mg orally twice daily or metronidazole 250 mg orally four times daily for 14 days (though some series treat for only 7 days). Second-line therapy has generally included colloidal bismuth 120 mg, tetracycline 500 mg, and metronidazole 250 mg all taken orally four times daily in addition to a PPI for 14 days. Several of the larger series with extended follow-up are summarized in Table 49.8. In most series, there is very prompt resolution of presenting symptoms including dyspepsia as well as nausea and vomiting. The complete response rate ranged from 50% to 95%, with higher response rates in series restricted to CS IE. H. pylori eradication is successful in >94% of patients when second-line therapy is included, although two courses of therapy were needed in 4% to 16% of cases. Delayed tumor response is a common feature of antibiotic therapy, with responses occurring at a median time to response of 3 to 4.6 months with a range of 1 to 45 months.
EUS has been used to help predict those patients with gastric MALT lymphoma that will respond to antibiotic therapy. EUS based on pretreatment patterns of involvement has been
evaluated in a number of small studies with limited follow-up (52,53,54,55,56). The initial pattern of gastric involvement determined by EUS was related to pathologic lymphoma response following eradication of H. pylori infection (Table 49.9). High rates of histologic remission were seen only in patients with T1m disease; T1sm or greater disease was associated with a low rate of complete response. EUS has not proven to be as accurate as biopsy in the determination of response; therefore, gastroscopy with biopsy is adequate for routine follow-up of gastric lymphoma (57,58).
evaluated in a number of small studies with limited follow-up (52,53,54,55,56). The initial pattern of gastric involvement determined by EUS was related to pathologic lymphoma response following eradication of H. pylori infection (Table 49.9). High rates of histologic remission were seen only in patients with T1m disease; T1sm or greater disease was associated with a low rate of complete response. EUS has not proven to be as accurate as biopsy in the determination of response; therefore, gastroscopy with biopsy is adequate for routine follow-up of gastric lymphoma (57,58).
Table 49.9 Relationship of T stage to response of gastric MALT lymphoma after eradication of HELICOBACTER PYLORI | ||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||
Given the current data, it is possible to select patients with gastric MALT lymphoma associated with H. pylori infection for primary treatment with antibiotics. Fully staged patients with CS IE1 have the highest likelihood of response. With EUS demonstrating disease confined to the mucosa, T1m should definitely be treated with antibiotics. However, as some patients with T1sm disease respond, using antibiotics in these patients is appropriate. Patients should have an upper endoscopy with biopsy every 3 to 6 months until remission, and then every 6 to 12 months for monitoring. Given the long time to treatment response, in the absence of symptoms or clear evidence of disease progression, monitoring disease with serial gastroscopy and biopsy is appropriate. Patients with autoimmune disease or tumor bearing the t(11;14) translocation have a low rate of response, and either should be referred for alternative treatment or monitored very closely after therapy with H. pylori eradication. A note of caution about considering these patients cured after antibiotic therapy is warranted based on a report of a large series of 86 patients who achieved a complete remission after H. pylori eradication in which 37% of patients recurred between 14 and 307 months; the high recurrence rate suggests the need for lifelong surveillance (59).
Eradication of MALT by Anti-H. pylori Therapy
In a large study of an unselected population with presentation of ulcer-like symptoms referred for gastroscopy, gastric MALT was seen in 70 of 151 patients with H. pylori infection whereas it was observed in only 5 of 49 patients without H. pylori infection (60). Thirty-eight of the MALT- and H. pylori-positive patients were treated with antibiotic therapy and underwent repeat gastroscopy 6 months later. Twenty-one patients had eradiation of the H. pylori and MALT. Twelve patients had persistence of both the H. pylori and MALT. Four patients had persistent MALT despite eradiation of the infection, and one had resolution of MALT with persistence of infection. MALT was persistent in a control group of 20 patients not treated for H. pylori. Thus, treatment of H. pylori infection presenting with concurrent MALT could prevent the development of gastric MALT lymphoma.
Gastric MALT Associated With Autoimmune Disease
Retrospective analyses have suggested that the presence of autoimmune disease is a predictor for poor response in early-stage gastric MALT lymphoma to H. pylori eradication (61,62). The impact of autoimmune disease was examined in a series of 22 patients with gastric MALT lymphoma, CS IE1, with H. pylori infection including 6 patients with autoimmune disease: Sjögren syndrome (3); polymyalgia rheumatica (1); autoimmune thyroiditis with psoriasis (1); and autoimmune thyroiditis (1) (61). All patients had successful eradication of the H. pylori infection; none of the 6 patients with autoimmune disease had a tumor response, whereas 15 of 16 control patients entered a complete response. In a follow-up study, the investigators examined 26 patients with concurrent Sjögren syndrome and MALT lymphoma (paraotid 14, orbit 2, submandibular 1, gastric 9) for translocation of the MALT1 gene (62). Six of the nine gastric MALT lymphomas bore the t(11;18) translocation, and one bore the t(14;18) rearrangement involving the MALT1 gene. This incidence of t(11;18) was higher than was seen in unselected series from the same institution (30%) (62). Alternation of the MALT1 gene has been associated with decreased response to antibiotics, potentially explaining the lack of response seen in patients with concurrent autoimmune disease and gastric MALT lymphoma. Although these data potentially suffer from selection bias as a consequence of the retrospective nature of these series, the data suggest an altered biology in patients with autoimmune disease. Prospective trials evaluating the impact of autoimmunity on the outcome of gastric MALT lymphoma are necessary.
High-Grade Gastric MALT Lymphoma
The series reviewed above included patients with the diagnosis of low-grade gastric MALT lymphoma characterized by diffuse infiltration by small- to medium-sized lymphoid cells. Some cases of gastric MALT lymphoma are distinguished by the presence of large transformed cells in clusters or sheets; these cases are often referred to as high-grade gastric MALT lymphoma. They are distinguished from DLBCL of the stomach by the persistence of LELs and cells with low-grade morphology (1). Early series based on very limited numbers of cases suggested that these were H. pylori independent and not responsive to antibiotics (2,10,16). However, antibiotic therapy
has been prospectively evaluated in the management of high-grade gastric MALT lymphoma in 16 patients with H. pylori-associated CS IE disease (63). In 15 patients, H. pylori infection was successfully eradicated, and 63% of the patients achieved a clinical complete remission (10 of 16, 63%) at a median of 3.9 months (1.5 to 17.7 months). At a median follow-up of 44 months, all the responding patients were alive and disease free. Patients with tumors penetrating into the muscularis propria had a poorer response (29%, 2 of 7) than did patients with mucosal or submucosal disease (100%, 4 of 4). Long-term follow-up updating the patients with high-grade MALT lymphoma were reported to have a persistent high rate of complete remission with H. pylori eradication of 58% (14 of 24); among the complete responders, no recurrences were observed after a median of 5 years of follow-up (64). Similar results have been reported in responses from 2 of 4 patients with high-grade gastric MALT lymphoma (65). In a third series of eight patients with high-grade gastric MALT lymphoma, seven patients had a complete remission after antibiotics; however, three patients received postremission therapy with surgical resection (1) and chemotherapy (2). Of five patients treated with antibiotics alone, four had ongoing complete remissions ranging from 6 to 66 months (median 13.5 months). Based on these limited data, patients with superficial high-grade gastric MALT lymphoma associated with H. pylori infection are candidates for eradication therapy. Patients need to be monitored closely, and if there is evidence of progression patients need to be referred for chemotherapy or radiation.
has been prospectively evaluated in the management of high-grade gastric MALT lymphoma in 16 patients with H. pylori-associated CS IE disease (63). In 15 patients, H. pylori infection was successfully eradicated, and 63% of the patients achieved a clinical complete remission (10 of 16, 63%) at a median of 3.9 months (1.5 to 17.7 months). At a median follow-up of 44 months, all the responding patients were alive and disease free. Patients with tumors penetrating into the muscularis propria had a poorer response (29%, 2 of 7) than did patients with mucosal or submucosal disease (100%, 4 of 4). Long-term follow-up updating the patients with high-grade MALT lymphoma were reported to have a persistent high rate of complete remission with H. pylori eradication of 58% (14 of 24); among the complete responders, no recurrences were observed after a median of 5 years of follow-up (64). Similar results have been reported in responses from 2 of 4 patients with high-grade gastric MALT lymphoma (65). In a third series of eight patients with high-grade gastric MALT lymphoma, seven patients had a complete remission after antibiotics; however, three patients received postremission therapy with surgical resection (1) and chemotherapy (2). Of five patients treated with antibiotics alone, four had ongoing complete remissions ranging from 6 to 66 months (median 13.5 months). Based on these limited data, patients with superficial high-grade gastric MALT lymphoma associated with H. pylori infection are candidates for eradication therapy. Patients need to be monitored closely, and if there is evidence of progression patients need to be referred for chemotherapy or radiation.
Influence of Regional Nodal on Response to H. pylori Eradication Therapy
Lymph node involvement rather than depth of invasion has been found to be the dominant factor in predicting outcome in gastric MALT lymphoma (66,67). Among 34 patients with H. pylori-associated localized gastric MALT lymphoma, CS IE and IIE, the complete remission rate was tied to lymph node involvement, being 56% (19 of 34) in patients with nodal involvement and 79% in patients without perigastric nodal involvement. Although depth of involvement was also associated with outcome, in the multivariate analysis only the presence or absence of nodal involvement predicted outcome. In another series of 48 patients, the response to antibiotics was found to be related to perigastric lymph node involvement as assessed by EUS rather than the pattern of tumor involvement in the stomach or to the histologic grade (67). Seventy-six percent of patients achieved a complete remission in the absence of perigastric lymph node involvement compared to 33% when EUS demonstrated lymph nodes (p = .025). Thus, nodal involvement determined by EUS is a predictor of poor outcome with H. pylori eradication therapy alone.
Table 49.10 Outcome of antibiotic therapy in HELICOBACTER PYLORI–negative gastric MALT lymphoma | ||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||
Role of Specific Translocation in Predicting Response
Cytogenetic abnormalities are relatively common in MALT lymphoma (68). A series of 252 primary MALT lymphomas were analyzed for translocations t(11;18)(q21;q21), t(14;18)(q32;q21), and t(1;14)(p22;q32), and for trisomies 3 and 18. These cytogenetic abnormalities were mutually exclusive and were found at a frequency of 14%, 11%, and 1.6%, respectively. Numeric abnormalities including trisomy 3 and/or 18 occurred in 42%. The cytogenetic abnormalities varied by primary site of MALT lymphoma: t(11;18)(q21;q21) was principally found in pulmonary and gastric lymphomas; t(14;18)(q32;q21) was most common in ocular adnexa/orbit, skin, and salivary gland lymphomas. Trisomies 3 and 18 each occurred most frequently in intestinal and salivary gland MALT lymphomas (68).
As shown in Table 49.10, 6% to 50% of cases of H. pylori-associated gastric MALT lymphoma did not respond to antibiotics. This finding may indicate an alternative etiology in these cases or evolution to antigen independence. The t(11;18)(q21;q21) translocation encodes a novel fusion protein between API2 and MALT1 that is most often seen in gastric MALT lymphoma (see Helicobacter pylori and Figure 49.1). A strong correlation has been observed between the presence of the API2-MALT1 transcript and resistance to treatment with antibiotics (18,19). A series of 111 patients with H. pylori-associated gastric MALT lymphoma were analyzed for the t(11;18) translocation, and the results were correlated to stage and response (19). Forty-three percent of the cases achieved a complete response, and 97% of these patients had CS IE disease. The API2-MALT1 fusion transcript was seen infrequently in responding cases (4%), whereas it was seen in 67% of the nonresponders including 60% of the patients with CS IE disease that failed to respond to H. pylori eradication.
The translocation can be detected by FISH (69). The assay uses two color probes, one derived from the API2 gene on chromosome 11 and the second derived from the MALT1 gene on chromosome 18 that can be applied to both interphase nuclei as well as metaphase chromosomes. The assay can be used on both fresh and archival tissue. Alternatively, the unique fusion transcript between the API2 and MALT1 genes can be
detected by reverse transcriptase–PCR (RT–PCR) (70). The assay uses internal API2 and MALT1 primers, and amplification results in a variable length product because the breakpoints on chromosome 18 occur at three locations; the breakpoint on chromosome 11 is consistent occurring between exons 7 and 8. This assay requires fresh tissue but is more sensitive for rare transcripts than is the FISH technique.
detected by reverse transcriptase–PCR (RT–PCR) (70). The assay uses internal API2 and MALT1 primers, and amplification results in a variable length product because the breakpoints on chromosome 18 occur at three locations; the breakpoint on chromosome 11 is consistent occurring between exons 7 and 8. This assay requires fresh tissue but is more sensitive for rare transcripts than is the FISH technique.
Table 49.11 Involved field radiation therapy for gastric MALT lymphoma | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree







