aTrials in which protocol biopsies were performed after transplantation: US study: days 7–28-360; Fisher et al.; only in HCV positive patients: month 12–24–36; Zervos et al.; month 12–18; Rolles et al.: days 5–10; Therapondos et al.: day 7; Martin et al.: month 3–12.
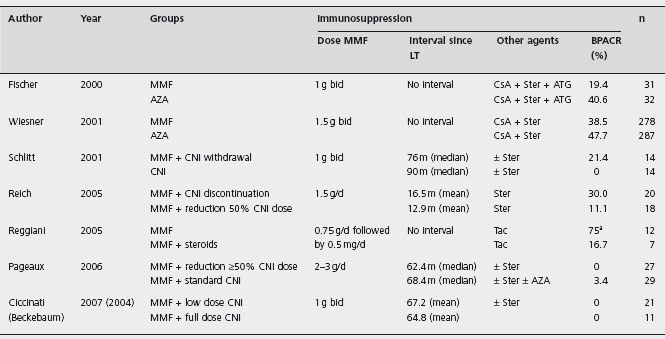
Tac: tacrolimus; CsA: cyclosporin; CsA-OB: cyclosporin, oily-based formulation; CsA-ME: cyclosporin, microemulsion formulation; AZA: azathioprine; MMF: mycophenolate mofetil; ATG: antithimocyte globulin; ALG: antilymphocyte globulin; BPACR: biopsy proven acute cellular rejection.
The five-year post-randomization surveillance of the Spanish multicenter RCT demonstrated no differences in overall survival (CsA-ME 76% vs Tac 66%, p = 0.18), re-transplantation (CsA-ME 8% vs Tac 10%, p = 0.73) or acute rejection rates (CsA-ME 33% vs Tac 26%, p = 0.8) [92]. However, the proportion of patients switched from CsA-ME to Tac was significantly higher (CsA-ME to Tac 29% vs Tac to CsA-ME 8%, p = 0.01), mainly due to lack of efficacy of allocated therapy. Both groups had similar occurrence of side effects, except for hyperglycemia, which was more frequent in the Tac group (CsA-ME 9.8% vs Tac 26.5%, p = 0.04), while CsA-ME had a higher rate of severe adverse events or prolongation of hospitalization (CsA-ME 94% vs Tac 64%, p < 0.05). In this study C0 monitoring was used.
However, in some of these trials, methodological aspects such as risk of bias in the sequence generation, baseline imbalance of the clinical factors, as well as the additional use of AZA in the CsA group and not Tac group, and the absence of defining diagnosis of rejection, suggest some caution in interpreting the magnitude of the difference between CsA and Tac.
In addition, several changes have occurred in the clinical practice since the design of these trials, such as the adoption of the C2 monitoring for CsA and the fact that AZA is now rarely used in primary immunosuppression regimens.
CNI-induced nephrotoxicity has a component of reversible renal vasoconstriction. Eventually tubulointerstitial chronic fibrosis and irreversible change result, but the interval between reversible and irreversible changes is variable [93]. Withdrawal of CNI during early stages of renal dysfunction results in improvement of renal function, but the optimal moment for conversion is not clear, although it is likely to be within six months of LT [94].
Therapeutic drug monitoring of cyclosporine Therapeutic monitoring of CNI is essential. CsA has a narrow therapeutic index, and an extremely variable pharmacokinetic profile and a strong pharmacodynamic linkage between desired and adverse effects.
An expert group has recommended C2 monitoring of CsA as optimal single-time point for monitoring [95]. LIS2T is an RCT that compared CsA-ME with C2 monitoring vs Tac [96], stratifying patients according to HCV status. Patients received either CsA-ME (n = 250) or Tac (n = 245) with steroids and some with AZA (41% CsA and 43% Tac). The primary outcome was the incidence of biopsy proven acute rejection within three months after LT: 26% CsA-ME vs 24% Tac (p = ns). The six-month survival rate was 89% (CsA-ME) vs 88% (Tac) (p = ns); the six-month graft loss rate was 4% (CsA-ME) vs 5% (Tac) (p = ns). The sub-analysis of patients receiving dual or triple therapy with AZA whether with CsA-ME or Tac showed similar acute rejection rates, also between HCV-positive and HCV-negative recipients. However, among HCV-positive patients death or graft loss at six months was significantly higher in the Tac group (15%) than in the CsA-ME group (6%) (p < 0.05). Tolerability and safety were comparable, other than a higher incidence of diabetes mellitus (14% vs 7%, p < 0.02) and diarrhea (29% vs 14%, p < 0.001) in patients treated with Tac. Patients undergoing living-donor transplantation (n = 39) in the LIS2T study showed similar results in terms of acute rejection, overall survival and graft loss between both treatments [97].
Thereafter, further surveillance in the LIS2T study with follow-up data available in 90% of patients, confirmed equivalent outcomes in trial groups as regards patient survival (85% CsA-ME vs 86% Tac), graft survival and rejection at 12 months after LT [98]. In HCV-positive patients, death or graft loss were more frequent in Tac patients (16% Tac vs 6% CsA-ME, p < 0.03), despite HCV recurrence being similar. However, the mean time to histological diagnosis of HCV recurrence was significantly longer with CsA-ME than with Tac (100 ± 50 days vs 70 ± 40 days; p < 0.05). More patients required medication for hyperglycemia in the Tac group, regardless of diabetic status at baseline.
Annual costs were lower for CsA-ME with C2 monitoring than for Tac ($5432 ± 2091 vs $8291 ± 3948 respectively, p = 0.001) in an RCT [99]. However, the annual pre-trans-plant and one-year post-transplant drug costs, and costs of concomitant medications were similar. Interestingly, this trial found no significant differences between Tac and CsA-ME-C2 therapies in terms of one-year patient survival, early acute rejection and incidence of metabolic disorders (diabetes mellitus, hypertension and hyperlipidemia). Opposite to the previous study, Shenoy et al. found that recurrent HCV occurred less frequently in Tac treated patients than in CsA-ME treated ones (21% vs 61%; p = 0.04), despite earlier recurrence (Tac 72 ± 42 days vs CsA-ME 145 ± 43 days, p = 0.006) [99]. These data from a single industry sponsored study show that CsA-ME-C2 provides at least equivalent immunosuppression to Tac, with similar costs, but the beneficial effects on HCV shown in the first study have not been substantiated [98].
Dosage of cyclosporine CsA is usually administered orally as two doses every 12 hours in LT patients. However, some patients have difficulty in achieving therapeutic C0 or C2 levels. To address this and to minimize adverse effects, conversion to once daily dosing of CsA-ME (75 ± 15 mg/day) was evaluated in 68 maintenance LT patients (4 ± 1.3 year after LT) with abnormal renal function [100]. The C2 levels ranged 748 ± 105ng/ml, without rejection episodes (although protocol biopsies were not done) and with improvement in renal function. Similar results were obtained in a non-randomized trial including 14 patients who underwent living-donor LT [101].
A randomized trial evaluated 60 post-LT patients with renal dysfunction to receive twice-daily CsA-ME vs conversion to once-daily equal dose vs conversion to once-daily reduced dose (to yield a C2 level 25–35% lower than the post-conversion C2) [102]. After conversion, C0 did not change, whereas C2 nearly doubled and the AUC increased by 29%. Moreover, once-daily dosing was associated with a trend to lower nocturnal mean arterial blood pressure. Thus, it has been suggested that reduction of 25–30% in the dose should be considered when conversion to once-daily dose is performed.
Therapeutic drug monitoring of tacrolimus Tac whole-blood trough concentration (C0) is routinely used to monitor therapy in most centres. As for CsA, C0 does not accurately reflect systemic exposure over the first 12 hours after dosing, for example patients with similar C0 Tac concentrations can have very different AUC due to the wide intra- and inter-subject variability in Tac pharmacokinetics [103]. Moreover, as for CsA there is no clear association between C0 concentrations and clinical outcomes. Clearance of Tac in patients in the early post-LT period is influenced by hematocrit and serum albumin, as well as concurrent medications such as diltiazem and fluconazole [104]. Thus, hypoalbuminemia and anemia, two of the most frequent clinical conditions in liver recipients, may increase the Tac clearance.
The relationship between the dose of Tac, trough concentration (enzyme linked immunosorbent assay, ELISA) and selected clinical endpoints (acute rejection, nephrotoxicity and other toxicities) were examined in a prospective multicenter study, which confirmed a poor correlation between the daily dose (mg/kg per day) and the steady-state whole-blood concentration. It suggested that to minimize nephrotoxicity without increasing the risk of rejection, it is necessary to maintain trough Tac blood concentration below 15 ng/ml [105].
In a recent study in which C0 was confirmed to be sub-optimal to monitor Tac therapy, C4 or Q were found the best single timepoints [106]. However this study was developed and validated in patients more than six months post-LT, and thus it cannot be extrapolated to the early post-LT period when the risk of rejection is greatest and when Tac pharmacokinetics may fluctuate widely.
To improve Tac monitoring, measurement of calcineurin activity (CNA) has been proposed, and evaluated in peripheral blood mononuclear cells from 14 patients at 0, 2, 3, 4, 6 and 9 hours after Tac intake on days 8, 21 and 90, post-LT [107]. The time of maximal inhibition of CNA was reached four hours after Tac intake; this could be a means to improve Tac monitoring during the early phase post-LT, but it is difficult to envisage widespread applicability.
Dosage of tacrolimus A multicenter four-period crossover study showed that conversion from Tac twice a day to a modified extended release (XL) formulation administered once-daily in the morning, led to equivalent exposure at a steady state [108]. There was less intrasubject variability with XL compared to Tac. The two-year post-conversion surveillance showed that the mean Tac whole blood trough concentration ranged from 6.2 to 6.6ng/ml over the two years after conversion and most patients did not require dose adjustment; patient and graft survival at was 98.6% with an acute rejection rate of 5.8% [109]. Adverse effects were similar to those observed in historical cohorts with twice-a-day Tac.
Tacrolimus rescue for acute cellular rejection Two studies have demonstrated that Tac at levels of 15–20mg/dl are effective as rescue therapy for steroid-resistant acute rejection in patients on CsA-based therapy [75, 110]. Moreover, a pilot study suggested that increasing Tac dosage (increments of 1–2 mg every one or two days with trough Tac blood levels of 15–20ng/ml), and continued low doses of steroids could be considered as treatment for early acute rejection episodes (biopsy proven), including severe grades of rejection [111]. B4 However, these higher doses of Tac (>15 ng/ml) are associated with renal dysfunction in the long-term, so that the cost-benefit of this strategy versus steroid therapy requires evaluation [105].
In summary, CNI-based immunosuppression, particularly Tac, improves the long-term graft survival in post-LT patients but renal dysfunction is frequent (9.5%) and this can worsen survival and lead to renal transplantation [13]. Reduction in CNI dosage often does not resolve this problem and suspension of CNI is often associated with increased rejection. Better use of CNI, and/or substitution with effective immunosuppressive agents without nephrotoxicity is required. The use of AZA, mycophenolate mofetil (MMF) and sirolimus with lower doses of CsA or Tac is the most commonly used strategy, as use of these agents as single drugs increases rates of rejection and graft loss [112].
Antimetabolites: azathioprine and mycophenolate mofetil
Azathioprine is a prodrug form of 6-mercaptopurine which inhibits T cell activation, reduces antibody synthesis and decreases the circulating monocytes and granulocytes [113]. AZA alone is relatively effective in the prevention of rejection but has very little effect upon an established immune response [114].
Mycophenolate mofetil is a selective inhibitor of the de novo pathway of purine biosynthesis, thereby providing more specific and potent inhibition of T cell and B cell proliferation than AZA. It has been used for both treatment and prevention of rejection in combination with CNI (see Table 42.3) [115].
aOnly trial in which protocol biopsies were performed (at day 7 after transplantation). This trial was stopped due to a high incidence of acute rejection in the study group.
MMF: mycophenolate mofetil; Ster: steroids; CNI: calcineurin inhibitors; Tac: tacrolimus; CsA: cyclosporin; AZA: azathioprine; ATG: antithimocyte globulin; BPACR: biopsy proven acute cellular rejection; LT: liver transplantation.
MMF (1–1.5 g twice daily) was superior to AZA (1–2 mg/kg/d) in preventing biopsy proven acute rejection in the first six months post-LT in two RCTs, but patient and graft overall was not improved [115, 116]. Ald Compared with AZA, MMF has fewer myelotoxic and hepatotoxic adverse effects, but has more gastrointestinal upset including diarrhea, which affects 30% of patients but usually resolves with reducing the dose [117]. The two agents should never be used together. MMF is teratogenetic and 3% of patients develop neutropenia [114]. Opportunistic infections are not significantly increased in comparison to AZA treatment. Monitoring of blood levels is not usually required. Retrospective analysis of large series of liver recipients (n = 15,133) reported better patient and graft survival, as well reduced late acute rejection rates in patients treated with MMF in addition to Tac and steroids, compared with Tac and steroids alone, again just showing that increased immunopotency results in less rejection [118,119].
An enteric-coated formulation of mycophenolate sodium (EC-MPS) has been developed to reduce the gastrointestinal side effects by delaying mycophenolic acid (MPA, the active metabolite of MMF) release until the small intestine. Bioequivalence has been shown in renal transplantation for both pharmacokinetics [120–122], and RCT [123]. In LT EC-MPS use is limited [124,125].
MMF has been successfully used as rescue therapy in 38 of 47 (80.9%) liver recipients with acute steroid resistant rejection, in 5 of 8 (62.5%) patients with chronic rejection, in 52 of 60 (86.7%) patients with chronic graft dysfunction and in 46 of 59 (77.9%) patients with CNI-related nephro-toxicity [126]. An RCT including 30 liver recipients who received Tac plus MMF vs Tac plus MMF plus steroids (MMF 750 mg bd for all), showed a higher rate of biopsy proven acute rejection in the former group (75% vs 17%, p < 0.002), with similar toxicity [127]. However early acute rejection did not affect graft or patient survival. As previously mentioned, this study unfortunately did not evaluate equipotent regimes in terms of immunosuppression and just showed that more immunosuppression results in less rejection.
To date there is no clear evidence that combination of MMF with CNI improves graft or patient survival compared to CNI and steroids or AZA. Its role may be more as a renal-sparing agent. When administered to reduce the CNI dose in patients with impaired renal function or as monotherapy, acute rejection episodes occurred in 9-38% of cases after CNI withdrawal [128–130], which was more frequent when withdrawal occurred within six months [131] or 12 months [132] after LT in two RCTs, with rates of 50% [131] and 60% [132]. Later withdrawal was safe in a retrospective review of 45 patients with renal dysfunction, treated at a median of 45 months after LT, either with MMF as monotherapy (n = 16), or in combination with low dose of CNI [133]. Therapeutic trough levels were deliberately kept < 5ng/ml or CsA < 50ng/ml. Acute cellular rejection was documented in only 6% with MMF mono-therapy, and serum creatinine values decreased, more so in the monotherapy group compared to the combination group. Similarly, another non-controlled but prospective study introduced MMF 7.7 ± 4.3 years after LT in 49 patients with CNI-associated chronic renal failure (14 Tac, 35 CsA) [134]. Creatinine clearance increased significantly after CNI reduction (from the baseline level of 42.9 ± 14ml/min to 48.8 ± 17ml/min after one year and 58.4 ± 20ml/minute after three years, p < 0.0001). No acute or chronic rejection was reported. B4
A trial randomized 28 post-LT patients on CNI treatment (CsA or Tac) with impaired renal function (defined as >20% decline in renal function with creatinine level 1.8-4mg/dl and/or creatinine clearance 20–60 ml/min) either to discontinuation or 50% reduction of CNI dose [129]. Both groups received MMF 1.5g twice daily and steroids. Improvement of 15% or more in glomerular filtration rate (GFR) occurred in 64% of patients with CNI suspension and in 50% of patients with CNI dose reduction, and GFR remained stable in 36% and 37% respectively in a per protocol analysis. Mild or moderate rejection occurred in 30% with CNI discontinuation and in 11% with CNI dose reduction. However, no comparison was made with full dose of CNI. The same strategy was evaluated in an RCT in 32 post-LT patients who received MMF followed by stepwise reduction of CNI (n = 21) or continued CNI therapy (n = 11) [135]. Serum creatinine levels and BUN decreased significantly with MMF at three months (p < 0.01) and GFR increased (p < 0.001), and the lipid profile, blood pressure and transaminase levels also improved after introduction of MMF. No rejection episodes were observed. Ald However, the follow-up for some patients was very short.
Pharmacokinetic data have shown that peak concentrations and bioavailability with IV MMF are more than twice those of oral MMF, which may provide immunological advantage, but this has not been evaluated [136, 137].
Therapeutic drug monitoring of MMF is effective in preventing acute rejection in kidney and heart transplantation [138, 139]. Some evidence suggests that MPA concentrations (its active metabolite) could be used for predicting acute rejection in LT recipients [140]. However, routine monitoring of MMF has not been widely performed and dose adjustments are usually performed based on adverse effects. It is common practice to use 2 g/day or less in some LT studies rather than the 3g/day in the renal transplant registration studies. There is great inter-individual variability of MPA pharmacokinetics, and as in the case of CsA and Tac, measurement after dosing of MMF may have a stronger correlation with AUC, than C0 monitoring [141–144]. Subtherapeutic values of MPA measured as C0 and AUC have been found in approximately two-thirds of patients during the first days after LT and in one-third at months 3 and 6 post-LT, but the clinical associations with this are unclear [145].
mTOR inhibitors: sirolimus and everolimus
These are inhibitors of the mammalian target of rapamycin (mTOR). Their immunosuppressive activity is primarily related to the blockade of interleukin-2 and interleukin-15 induction of proliferation of T and B cells, and their syner-gism with other drugs such as CsA, Tac or MMF allows new combinations of therapy (see Table 42.4).
Cell growth and angiogenesis are also linked with mTOR activity; mTOR inhibition decreases HCC growth [146]. Recurrence of HCC after LT has become an important problem due to a longer recipient survival under chronic immunosuppression. Data from uncontrolled clinical studies and case reports suggest mTOR inhibitors may delay onset or reduce recurrent HCC [147–149]. A large randomized trial is taking place to clarify this issue (clini-caltrials.gov identifier NCT 00328770).
Sirolimus Sirolimus (rapamycin) is a macrocyclic lactone with similar structure to Tac, but its mechanism of action and adverse effect profiles are quite different. Sirolimus (SRL) blocks signal transduction in T-lymphocytes and inhibits cell-cycle progression from G1 to S phase, but does not inhibit calcineurin [150,151].
While AZA and MMF often are used in conjunction with CNI inhibitors, since their use as single agents increases rates of rejection and graft loss [112], SRL is a promising alternative that may be equivalent to CNI in preventing graft rejection [152–154]. The adverse effects of SRL include dose-dependent hyperlipidemia, thrombocytope-nia, anemia, leukopenia, with the absence of neurotoxicity, nephrotoxicity and diabetogenesis, but it has adverse effects on wound healing [155]. Risk of oligospermia in young male patients has also been reported [156].
Sirolimus as primary immunosuppression An international trial comparing SRL with Tac and steroids (n = 110) vs Tac and steroids alone (n = 112) in LT patients was suspended in phase II due to an increase of hepatic artery thrombosis in the SRL group (5.5% vs 0.9%) [157]. This has not been seen in non-randomized studies, but a formal warning remains on the drug information sheet [158,159].
The first report only comprised 15 patients treated with SRL alone, or SRL plus CsA-ME, or SRL plus CsA-ME plus steroids [160]. Patients on triple therapy had no rejection, while 28% and 75% of those with dual and monotherapy, respectively had rejection. A retrospective study compared three groups: SRL alone (n = 28), SRL plus CIN (n = 56) and CNI alone (n = 101) [161]. One-year patient and graft survival rates and histologically proven acute cellular rejection rates (no protocol liver biopsies) were not significantly different. The mean creatinine at one month was similar despite being higher in the SRL group at the time of transplantation.
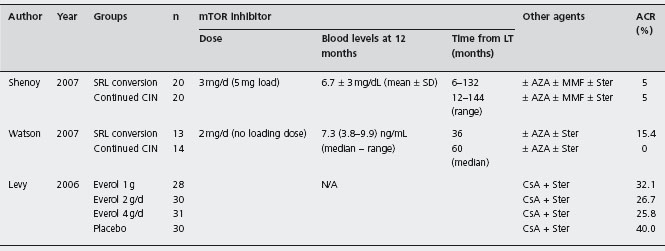
Protocol biopsies were not performed in any of the three trials. In the Levy et al. trial, histological diagnosis of rejection was required, but not in the Shenoy et al. trial. The definition of ACR was not stated in the Watson et al. trial.
SRL: sirolimus; Everol: everolimus; MMF: mycophenolate mofetil; Ster: steroids; CNI: calcineurin inhibitors; CsA: cyclosporin; AZA: azathioprine; ACR: acute cellular rejection; LT: liver transplantation.
Trotter et al. reported that low dose SRL plus CNI and minimal dose of steroids (three-day taper), resulted in less acute rejection rates than historical controls (treated with CNI plus 14-day tapered prednisone (30% vs 70%; p < 0.01) [162]. Steroid-resistant rejection decreased by 90%. B4 However, as in many studies the interpretation of these data is difficult as no protocol biopsies were done. McAlister et al. reported 56 patients who received low-dose Tac and SRL (target: trough levels, 5 and 7ng/ml respectively) with prednisone up to six months after transplantation [159]. The biopsy proven acute cellular rejection rate was 14%, approximately 50% lower than historical controls. No patient had steroid-resistant rejection. Pridohl et al. reported patients transplanted for acute liver failure with triple immunosuppression (SRL, Tac and steroids); acute rejection and steroid-resistant rates were 14% and 0% respectively [163]. Some authors have suggested that SRL should be used in combination with low dose Tac to minimize adverse effects from either drug, whilst avoiding an increased risk of rejection [164,165].
Dunkelberg et al. reported 170 LT recipients treated with SRL (2mg/day) as part of primary immunosuppression [158]. One-year patient (93%) and graft survival rates (92%) were not different from historical controls (95% and 89% respectively). The acute rejection rate and use of OKT3 (monoclonal antibody against CD3, muromonabCD3) was significantly lower in SRL-treated patients (14%) versus controls (39%), similar to a previous report [162]. However, the proportion of patients needing OKT3 was very high, both in the controls and SRL-treated groups, so that the precise immunosuppressive endpoints are difficult to compare with other publications. Hepatic artery and wound complications in SRL-treated patients were similar to historical controls.
Further studies are needed to assess the value of SRL as primary immunosuppression after LT, either as a single agent or in combination with other agents.
Conversion from CNI to sirolimus Chronic renal dysfunction occurs in approximately 18% of LT recipients by five-year post-LT and several patients develop end-stage renal disease which shortens patient survival [14]. In contrast to CNI, neither nephrotoxicity nor neurotoxicity has been described with SRL [155], so that it has been used in liver recipients with renal dysfunction, in order reduce or stop CNI, as well as for neurotoxicity or chronic rejection.
The first RCT assessing CNI withdrawal and replacement with SRL allocated 40 liver recipients with CrCl between 40–80ml/min, to receive either SRL or continued CNI [166]. The mean SRL concentrations at one, three and twelve months were 9 ± 3.1; 7.7 ± 1.9 and 6.7 ± 3mg/dl respectively, with a mean dose throughout of 3mg/day. Early improvement in CrCl was seen at one and three months in SRL patients: 75ml/min SRL vs 61 ml/min controls, p = 0.024 at one month and 75ml/min SRL vs 56 ml/min controls, p = 0.012 at three months. However, at 12 months the difference was not significant: 72 ml/min SRL vs 58ml/min controls, p = 0.09. Ald This could be because the conversion was too late, at a mean of 4.4 years after LT. The incidence of acute rejection was 5% in both groups and there was no significant difference in lipid profile.
Similarly, another RCT allocated 27 liver recipients with GFR < 65ml/min, to remain on CNI therapy (86% Tac, 14% CsA) or to switch to SRL based immunosuppression [94]. Renal function was measured by the change in 51Cr-ethylenediaminetetraaceticacid GFR (delta GFR). The median time from LT to randomization was three years (0.88–7.4). The median whole blood SRL concentrations were 6.3 ng/ml at three months and 7.3 ng/ml at 12 months. Tac median 12-hour trough concentrations were 7.7 at three months and 7ng/ml at 12 months. Mean CsA concentrations at three and twelve months were 86 and 107 and 147 ng/ml. Conversion to SRL led to a modest but significant improvement in renal function at three months after conversion, but the difference was not significant at one year. Ala No significant differences in serum creatinine were found. No acute rejection was diagnosed in patients with CNI, while it occurred in two patients (15%) on SRL. Adverse effects were similar except for rash and hyper-cholesterolemia requiring new statin therapy in the SRL group.
These data suggest that conversion to SRL can be done safely and provide adequate immunosuppression without increased incidence of rejection, graft loss or infection in LT recipients. However, there is no evidence as yet for long-lasting renal improvement. Moreover it is not clear if Tac dosing was reduced to a minimum as therapeutic concentrations could still be measured in blood. Multicenter randomized trials including larger groups, in particular, with much earlier conversion intervals, minimal Tac dosing, as well as longer follow-up, are needed to address the benefit of conversion to SRL. Lastly, the hypercholeste-rolemia induced by SRL may affect cardiovascular risk despite use of statin agents.
Everolimus Everolimus is a rapamycin derived compound with improved bioavailability and shorter half-life than SRL (18-35 hours vs 60 hours). Experience with everolimus in liver recipients is more limited than that with SRL. It has lower rates of acute rejection than those seen with AZA in heart transplants and similar to MMF in kidney transplants and may result in less cytomegalovirus infection [167,168]. It inhibits the growth of human Epstein-Barr virus-transformed B lymphocytes in vitro and in vivo, so it may be optimal when treating post-transplant lymphoproliferative diseases or for prevention [169]. CsA increases exposure to everolimus.
Levy et al. performed a randomized placebo-controlled trial evaluating everolimus combined with CsA (target trough level of 150–400ng/ml) and prednisone in liver recipients [170]. Three different doses of everolimus were compared: 0.5 mg bid (n = 28) vs 1 mg bid (n = 30) vs 2 mg bid (n = 31) vs placebo (n = 30). Biopsy proven acute rejection episodes were: 39.3% (0.5mg); 30% (1.0mg); 29% (2mg) vs 40% placebo, all non-significant. Rejection was three-fold higher when levels were = 3 ng/ml. Overall graft and patient survival rates and renal dysfunction and its severity were similar across groups. However, the small sample size and high drop-out rates prevent robust conclusions. Larger RCTs are required.
Anti-CD3 pan-T cell (orthoclone OKT3)
Muromonab–CD3 (OKT3) is a monoclonal antibody with defined specificity to the CD3 receptor of T cells, thus inactivating both naive and activated cytotoxic T cells. It has been used to prevent acute rejection and to reduce CNI dosage [171, 172]. It was the first monoclonal antibody preventing liver graft rejection. Re-exposure to OKT3 can result in lower efficacy due to the development of antimurine antibodies. There is increased risk of developing post-LT lymphoproliferative disorders and its use is associated with worse recurrence of HCV.
Randomization of 52 LT patients to receive either CsA + AZA + steroids or AZA + steroids + OKT3 followed by conversion to CsA at 14 days, showed lower incidence of acute rejection within two weeks after LT (41% vs 72%, p < 0.02) as well as better renal function in the OKT3 group [173]. Nevertheless, long-term follow-up showed similar rejection rates at 30 days post-LT and similar renal function at 6,12 and 24 months [174]. Ald A trial comparing OKT3 (n = 44) vs a IL-2 receptor antibody (LO-Tact-1) (n = 43) vs no induction (n = 42) in liver recipients on treatment with CsA, AZA and steroids, showed that both agents significantly reduced acute rejection with fewer CMV infections in the LO-Tact-1 group [175]. These results, as well as improved use of current immunosuppressives, make OKT3 less likely to be used as induction therapy.
Interleukin-2 receptor blockers: daclizumab and basiliximab
T-lymphocytes play a central role in the initiation and progression of the rejection response. Activated T-lymphocytes secrete IL-2 that acts in an autocrine and paracrine fashion to drive the response forward, and produces more IL-2 receptors (IL-2R). As only activated T-lymphocytes express IL-2R, blocking this receptor with a monoclonal antibody provides a highly selective approach to prevent rejection [176]. Daclizumab and basiliximab are chimeric and humanized antibodies that act on a receptorial subunit, which is expressed only on activated T-lymphocytes, thus selectively inhibiting their proliferation. However, due to some redundancy within the IL-2R complex, which permits the IL-2R signalling despite the use of these antibodies, they have to be used together with other agents, such as CNI (see Table 42.5).
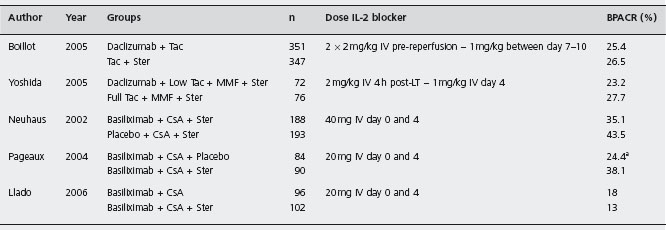
aOnly trial in which protocol biopsies were performed (at month 12 after transplatation).
MMF: mycophenolate mofetil; Ster: steroids; CsA: cyclosporin; AZA: azathioprine; BPACR: biopsy proven acute cellular rejection; LT: liver transplantation.
A pilot study evaluated the daclizumab (1 mg/kg IV, immediately pre- and post-operatively and four more doses at two-week intervals), combined with MMF and steroids, without use of CNI [177]. The study was stopped after the first seven liver recipients due to a 100% incidence of acute rejection, with 57% being steroid resistant. In contrast, retrospective analysis of 25 patients receiving the same therapy but with CNI had a rejection rate of 36% [24].
Considering the sequence of events in graft rejection in LT [24], immunosuppression with IL-2R blockers could play a role at the very beginning, leaving the introduction of CNI to a point when these agents can be introduced without excessive risk of infection or renal dysfunction, that is, monotherapy with IL-2R blockade for the first to second week and then monotherapy with CNI or other drugs.
Daclizumab A multicenter randomized trial assessed daclizumab as a steroid avoiding agent in 347 patients receiving Tac plus steroids against 351 patients on steroid-free regimen with Tac following daclizumab induction (follow-up three months) [178]. Acute rejection proven by biopsy that required treatment was similar, as well as graft and patient survival. The daclizumab group had less cor-ticosteroid-resistant acute rejection (6.3 vs 2.8% respectively, p = 0.027), less diabetes mellitus and CMV infection, without increased renal toxicity. Ald
In a multicenter, pilot study, 102 liver recipients were treated with daclizumab (2 mg/kg within six hours following transplant and 1 mg/kg on day 7), MMF and Tac [179]. Acute rejection proven by biopsy was 9.8% at six months (none graded as severe) and 11.8% after 12 months. Patient and graft survival rates at six months were 96% and 95% respectively. Infections, hypertension, diabetes mellitus and hypercholesterolemia occurred in 22%, 37%, 14% and 2% of patients respectively. B4 Thus, a steroid-free regimen using daclizumab, MMF, and Tac may prevent the occurrence of acute rejection without decreasing safety.
An RCT compared renal function in liver recipients treated with daclizumab (2 mg/kg IV within four hours postoperatively and 1 mg/kg IV on day 4 postLT) and delayed low-dose Tac (starting day 4–6) (n = 72), vs those treated with standard Tac regimen without induction (n = 76) [180]. Both groups also received MMF and tapering steroids. The GFR was significantly better in the daclizumab group during the first week after LT, but this was not maintained during the 12 months follow-up. Ald Patient survival and acute rejection rates were similar. Therapy with daclizumab and delayed CNI may not have long-term benefits.
A large series of 209 LT patients receiving daclizumab induction (2 mg/kg intraoperatively and 1 mg/kg on day 5 post-LT) was compared with 115 patients without induction therapy [181]. Despite the delayed initiation of CNI in the daclizumab group, there was less acute rejection within six months (25% vs 39%; p = 0.01). Moreover, patients on daclizumab with pre-LT creatinine levels >1.5mg/dl showed sustained improvement of renal function, while it worsened in those without daclizumab. However patient and graft survival and the chronic rejection rates were not significantly different, again questioning whether long term benefit accrues. B4 Currently, daclizumab has been withdrawn from the market.
Basiliximab Calmus et al. evaluated 101 patients treated with basiliximab (20 mg dose on day 0 and 4) in conjunction with CsA (adjusted according to therapeutic levels), AZA (1.5mg/kg per day), and steroids (tapering from 200mg/day to 5mg/day) [182], and compared these with a historical cohort derived from Multicentre International Study in Liver Transplantation of Neoral (MILTON) [183]. One-year patient and graft survival rates were 90.1% and 88.1% respectively. Biopsy-confirmed ACR with basiliximab was 22.8% and none was severe. Malignancies, infections or other adverse effects were not increased. Rejection episodes were more frequent in HCV positive compared to HCV negative patients (29% vs 20%, p = ns). B3 Basiliximab in association with Tac and steroids similarly prevented ACR in live donor liver recipients, with a rejection-free probability of 93.5% within three months and 93.8% patient survival rate at three years [184, 185]. Neuhaus et al. reported an RCT comparing basiliximab (n = 188) or placebo (n = 193) in addition to CsA and steroids [186]. There were no protocol biopsies, but clinically suspected rejection was confirmed by liver biopsy. Within six months, acute rejection rates were 35.1% basiliximab and 43.5% placebo (p = ns). Rejection rates in HCV positive patients were slightly more in basiliximab treated patients (39.1% vs 36.2%, p = ns). Complications including infections were similar. Ald The greater rejection rates in HCV positive patients might result from false positive reporting linked to histological similarities between recurrent hepatitis C and acute rejection [187]. However, caution is needed in HCV infection, as adjuvant IL-2R antibodies combined with MMF in the early peritransplantation period was associated with early recurrence of hepatitis C and more rapid histological progression of disease in another study [188].
An RCT evaluated 198 liver recipients (45% HCV-positive) treated with basiliximab as induction therapy with CsA as maintenance immunosuppression, 102 of whom received steroids and 96 were steroid-free [189]. Biopsy-proven ACR, patient and graft survival, and infection rates were similar, including in HCV versus non-HCV subgroups. Diabetic patients in the steroid group had significantly more bacterial infections. The incidence of de novo hypertension and hypercholesterolemia was comparable at six months. Ald
In another multicenter trial treated initially with basiliximab, CsA and steroids were allocated to receive maintenance therapy either with CsA and steroids (n = 90) or steroid withdrawal at day 14 (n = 84) [190]. Biopsy-confirmed rejection and treated acute rejection by six months was higher in the steroid-free group (38.1% vs 24.4%, p = 0.03). Metabolic complications were similar. These results were also similar at 12 months follow-up. Acute rejection was easily controlled in most patients and did not affect graft survival. Ald
Non-randomized studies suggest basiliximab induction may allow delay in introducing CNI and reduced CNI dosage, thus improving renal function as well as reducing acute rejection. A prospective study included adult living donor LT (LDLT) recipients assigned to either basiliximab induction (Tac delayed until renal function improved; n = 27) or conventional immunosuppression without basiliximab (Tac started on the first day post-LT; n = 18) [191]. Patients in the induction group were in worse condition pre-LT and had a higher blood loss and transfusion. The serum creatinine levels at the second and third months post-LT were lower in the induction group (p < 0.05). Renal insufficiency (an increase of = 0.5mg/dl in serum creatinine over the baseline value, or an increase of >50% over baseline, or a reduction of 50% in calculated creatinine clearance, or need for dialysis) at the third month post-LT was lower in the induction group (26% vs 67%, p = 0.007). Acute rejection at six months, as well as infections and diabetes mellitus were similar, and the median cholesterol level was lower in the induction group (152mg/dl vs 196mg/dl, p = 0.03). B3 However, the lack of randomization, the small number of patients and the short follow-up, negate robust conclusions. The same considerations pertain to 31 patients (94% chronic hepatitis B carriers) treated with basiliximab with steroid withdrawal 24 hours after LT and reduction of Tac dosage, which showed similar findings compared to historical controls [192].
Anti-thymocyte globulin
Anti-thymocyte globulins are heterologous preparations consisting of polyclonal anti-lymphocyte antibodies. A rabbit derived polyclonal antibody (RATG) was shown to be an effective immunosuppressive agent in LT [193]. The role of RATG in LT has been evaluated as a CNI sparing agent [194], or to delay CNI administration in patients with renal dysfunction [195], to reduce or eliminate steroid use [196], or to avoid maintenance immunosuppression [197]. Moreover, its ability to selectively deplete T cells has been used for both prophylaxis and treatment of acute cellular rejection per se.
In conjunction with steroids, RATG reduced rejection without increasing infectious complications or malignancy [193,198]. Eason and colleagues randomized 71 liver recipients to induction therapy with either RATG (n = 36) or steroids (n = 3 5) with maintenance Tac and MMF, with or without prednisone [196]. The rate of biopsy proven rejection was not significantly different: 20.5% RATG vs 32% steroids. However, the RATG group had less rejection needing steroids (p = 0.01), without increased complications, in particular cytomegalovirus infection, diabetes and HCV recurrence. A follow-up of these patients and data on additional patients undergoing steroid-free OLT confirmed these results [199]. Ald
Starzl et al. reported using RATG (5mg/kg) as pre-treatment therapy in 82 solid organ transplant recipients (17 LT) followed by minimum use of post-transplant immunosuppression (Tac monotherapy during the first four months, then spaced Tac therapy) [198]. Graft survival rates were 89% at one year and 88% at 13–18 months. RATG enabled 57.5% of recipients with surviving grafts to be maintained with spaced doses of Tac monotherapy from four months. B4
A retrospective analysis of 198 liver recipients, 118 receiving RATG and delayed initiation of CNI, and 80 receiving no antibody and early initiation of CNI, showed that delayed CNI initiation with RATG significantly improved serum creatinine levels and GFR, with similar patient and graft survival [200]. Overall infection and cytomegalovirus infection rates were significantly lower with RATG, with less early biopsy-proven acute rejection (16% vs 26%, p = 0.08). Another comparison between 129 liver recipients with CNI immediately after LT vs 262 with CNI after initial short-term RATG induction, showed that by one year acute rejection rates were significantly lower in RATG treated patients (14.5% vs 31.8%, p = 0.0008) [201]. Again, serum creatinine levels and GFR were better in the RATG group, with no additional harmful effects. Undesired side effects occurred at a similar rate in both groups. B3
Use of a three-day ATG induction therapy regimen after LT has been promoted as it results in no more rejection than a ten-day course, while reducing the adverse effects, especially lethal infection [202].
Anti-CD52 (alemtuzumab)
New protocols of tolerogenic preconditioning have been developed using alentuzumab (Campath-1H) and low doses of CNI. Alentuzumab is a humanized monoclonal antibody directed against the CD52 antigen, a pan T, B and natural killer cell, and monocyte markers [203]. It rapidly depletes lymphocytes, monocytes and other cells without affecting neutrophils and hematopoietic stem cells. The depleted cells begin to re-emerge gradually during a period of six months without returning to baseline levels. This activity is believed to prevent an aggressive lymphocytic immune response after transplantation and allow a more gradual engagement of the host immune system under low conventional immunosuppression. This regimen of host conditioning prior to transplantation, followed by minimum post-transplant immunosuppression could increase the chance of developing tolerance [21, 22, 204-207].
The most extensive experience with the use of alemtuzumab in solid organ transplantation has been done in kidney transplantation [17, 208], as well as intestinal and multivis-ceral transplantation [209], in which treatment with Campath-1H and low dose of CNI has shown encouraging results.
The outcome of 77 liver recipients treated with Campath-1H and low dose Tac, with steroid-free maintenance, compared with 50 patients treated with Tac and steroids (HCV patients excluded), showed no differences in patient and graft survival, while rejection-free survival was higher in the Campath-1 group: 51% vs 65% at 12 months, p = 0.009 [23, 210]. Tac-related nephrotoxicity and mean creatinine levels were higher in the control group (p = 0.0001 and p < 0.05 respectively). B3 No differences in viral infection rates were found. Thus, Campath-1 may be at least as effective, as Tac and steroid regimens, with less renal toxicity, but long-term follow-up is needed, especially with regard to risk of malignancy.
A similar study evaluated 76 liver recipients (50% HCV positive) treated with Campath-1 induction and low-dose Tac compared to 84 patients (31% HCV positive) receiving standard immunosuppression [211]. No differences in rejection or patient and graft survival were found at one year. However, HCV positive patients did significantly worse than those who were HCV negative, both in the induction and the control group. Moreover increased HCV viral replication was worse with Campath-1. B3 Until now, optimal dose of Campath-1 and ideal combinations of maintenance immunosuppression remain to be determined and the effect on HCV recurrence documented. Its use as a primary treatment for rejection has not been studied. Randomized-controlled trials addressing these issues need to be performed.
Ursodeoxycolic acid
Ursodeoxycholic acid (UDCA) is a hydrophilic bile acid which may improve parenchymal function in cholestatic states and possibly slow the progression of primary biliary cirrhosis [212, 213]. Additionally, UDCA reduces the expression of major histocompatibility complex (MHC) class I antigens in patients with primary biliary cirrhosis and therefore may have immunomodulatory effects on T cell-dependent liver damage [212–214]. During acute cellular rejection, MHC class I and II antigens are expressed on hepatocytes, although these are not primary target cells. Cholestasis itself may induce an increased expression of MHC class I antigens on hepatocytes, which can lead to lymphocyte CD8+-dependent cytotoxicity [215]. Based on these theoretical considerations, UDCA might decrease acute cellular rejection episodes in LT patients.
There are seven randomized controlled trials involving 335 liver recipients which evaluated UDCA or tauro-UDCA for prevention of acute allograft rejection (see Table 42.6) [216-222].
All seven trials failed to demonstrate that UDCA prevented the occurrence of acute rejection, confirmed by a meta-analysis of six trials [223]: acute cellular rejection (RR 0.89; 95% CI: 0.74–1.08), mortality, re-transplantation or steroid-resistant rejection were not improved. Ala
Table 42.6 Randomized trials on ursodeoxycholic acid in liver transplanted patients included in the meta-analysis.
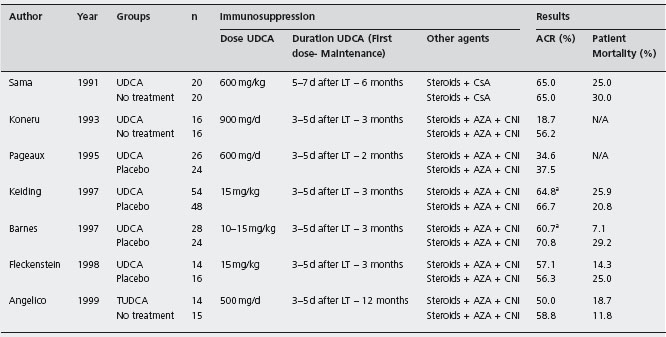
aTrials in which protocol biopsies were performed after liver transplantation: Keiding et al.: days 7-21-90-365; Barnes et al.: day 7 and month 3. UDCA: ursodeoxycholic acid; TUDCA: tauro-ursodeoxycholic acid; CNI: calcineurin inhibitors; CsA: cyclosporin; AZA: azathioprine; ACR: acute cellular rejection.
Assy et al. [224] performed a further RCT evaluating whether UDCA decreased rejection after total immunosup-pressant withdrawal, in 26 liver recipients, free of rejection while on immunosuppressive agents for a minimum of two years after LT. They were randomized to UDCA (15mg/kg) (n = 14) or placebo (n = 12) followed by sequential withdrawal of their immunosuppression over several months. Rejection and its severity were no different: UDCA 43% vs control 75% (p = 0.09) nor in the severity of the rejection. Ald
Granulocyte-colony stimulating factor
Studies in animal models as well as clinical trials have demonstrated significant benefits of human recombinant granulocyte-colony stimulating factor (G-CSF) for the treatment of infections in bone marrow recipients, while the experience in solid organ transplantation is more limited. G-CSF used for the first 7–10 days resulted in less acute rejection in 37 liver transplant recipients, compared with historical controls (n = 49) receiving the same immunosuppressive protocol (22% vs 51%). This could be related to a significant reduction in serum TNF levels, which may be a key player in allograft rejection [225, 226]. However, a subsequent multicenter randomized placebo-controlled trial comprising 194 patients did not confirm reduced infection rates and biopsy proven rejection was more common in the G-CSF treated group compared with placebo (30% vs 19%) [227]. Ald
New immunosuppressive agents
Leflunomide is a member of malononitrilamide family which targets the de novo pathway of pyrimidine biosynthesis and thus inhibits T and B cell proliferation. A retrospective review of the use of leflunomide was carried out in 8 liver and 45 kidney transplant recipients [228]. CNI were stopped completely in four liver recipients and reduced by 65% in another patient. No evidence of acute rejection developed in any of these liver (or kidney) transplant patients.
FK778, a synthetic derived from leflunomide, has been developed to reduce the extended half-life of leflunomide (6–45 hours vs 15–18 days) [229]. Studies in LT are awaited.
FTY 720 is a unique immunosuppressive agent that not only inhibits lymphocyte proliferation, but also results in a redistribution of lymphocytes into lymph nodes and out of circulation. This ability of FTY 720 to impair effector T cell homing is achieved without affecting induction or expansion of memory responses, suggesting that it may leave tolerizing interactions intact [230]. Studies in human LT are awaited.
Janus kinases (JAK) are intermediaries between cytokine receptors and STAT, which result in activation of the immune cells. JAK-3 may be an excellent target for clinical immunosuppression due to its requirement for signaling by multiple cytokines. JAK3/STAT inhibitors in animal models lead to immunosuppression to prevent rejection, without inducing many of the side effects observed with other current therapies [231]. Human studies are awaited.
The future immunosuppression in liver transplantation
Future immunosuppressive strategies will be designed to help the development of tolerance of the allograft, selectively stimulating and/or minimally suppressing the recipient’s immune reaction. Studies of the current immunosuppressive agents suggest two ways in which this goal might be attained. First, CNI dosage minimization or even avoidance will continue to be the focus of future studies, to avoid adverse effects and too much rejection. Second, use of new selective immunosuppresive agents which do not depress tolerizing mechanisms or even enhance them, while avoiding side effects, need to be developed [21, 22]. Both approaches need an easy method to assess immuno-potency of the immunosuppressive agents and/or a measure of tolerance. The surrogate markers of blood concentrations of immunosuppressives in current use relate more to avoiding toxicity than to issues of rejection or tolerance. These new assays would allow personalized medicine, by individual tailoring doses of immunosuppressive therapy, depending on “day-to-day” immune status.
Weaning immunosuppression
Steroid withdrawal and steroid avoidance
Steroids have been the cornerstone of immunosuppressive regimens since the inception of solid organ transplantation. In the past two decades immunosuppressive protocols have evolved driven by a deeper understanding of immu-nological events after transplantation, and more focus has been placed on minimizing the multitude of side effects and toxicities associated with a long-term therapy with steroids (renal function, infectious risk, loss of bone density, diabetes, hypertension, hyperlipidemia, etc.). The trend has been to use fewer steroids for maintenance therapy. In 2000, a series showed safe withdrawal from the third postoperative month, but the duration of steroid use after LT and the potential role of complete steroid avoidance remain uncertain [232].
Studies evaluating steroid-withdrawal and steroid avoidance in LT are heterogeneous, especially with regards to the interval at withdrawal (1 day–9 months). The majority of protocols utilize regimens based on MMF in conjunction with Tac and the proportion of studies using CsA is lower compared to those using Tac. However, many protocols utilize antibody induction agents such as daclizu-mab, basiliximab and rabbit-derived antithymocyte globulin, thus increasing immunopotency.
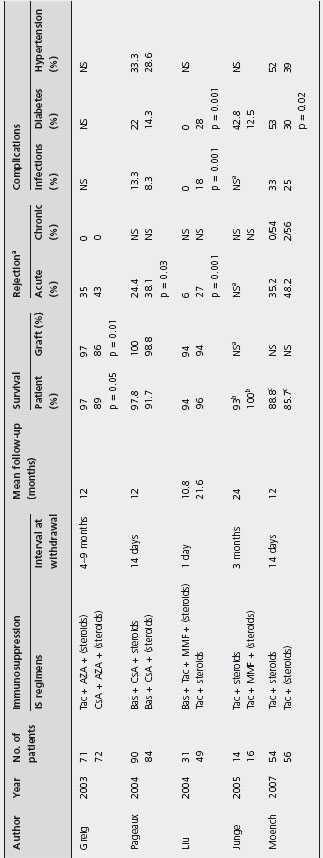
Steroid withdrawal Steroid withdrawal based on Tac-containing regimens showed comparable patient and graft survival to protocols using maintenance immunosuppression with steroids; one study found that histology-proven acute rejection was less with steroid withdrawal compared to a steroid regimen (6% vs 27%; p = 0.001) [192, 233, 234]. Infection was less amongst patients who underwent steroid withdrawal (0% vs 18%; p = 0.001) and two studies showed significant reduction in the onset of diabetes mellitus amongst patients who stopped steroids (Table 42.7) [192, 234]. B3
Pageaux et al. evaluated the impact of steroid withdrawal based on a CsA-based regimen in 174 patients, randomized to receive CsA and prednisolone (n = 90) or CsA and placebo (n = 84) [190]. Histologically proven acute rejection at six months was significantly more frequent among patients without steroids than patients on prednisolone (38.1% vs 24.4%; p = 0.03). However, patient and graft survival were similar between the two group, as well as infection rate, diabetes and hypertension. Ald
Greig et al. evaluated the safety and efficacy of early steroid withdrawal, comparing 71 patients treated with Tac to 72 patients treated with CsA-ME. They found that patients on Tac had a statistically significant better patient and graft survival (97% and 97%) compared to patients on micro-CsA (89% and 86% respectively) [86]. Acute rejection occurring during the first year was similar (35% with Tac and 43% with micro-CsA), and no chronic rejection developed during this period, presumably because of the short follow-up. B4
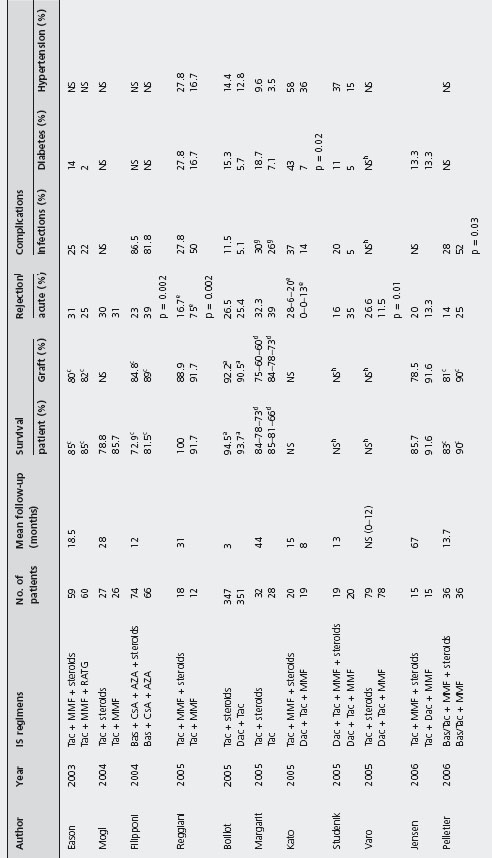
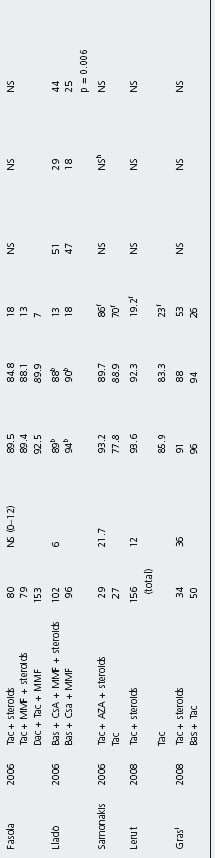
Steroid avoidance Several studies have evaluated the safety and efficacy of steroid avoidance in liver transplanted patients (see Table 42.8) [127,178,189,196, 235–247].
No study found statistically significant differences in patient and graft survival comparing steroid-free and steroid inclusive regimens, but these studies are heterogeneous with a range of evaluation time between three months and five years, and often evaluated increased immunosuppression in the steroid free groups. B4
Two studies found statistical differences in biopsy-confirmed acute rejection rates [127, 235]: 24.3% with steroids vs 39.4% without (p = 0.04) [235], and 16.7% steroids vs 75% no steroids (p = 0.002) by Reggiani et al. [127]. In contrast, Varo et al. reported patients on steroids having a higher acute rejection rate compared to patients on steroid-free regimen (11.5% vs 26.6%; p = 0.01) [240].
aData non specified, but no difference between the two group was reported.
bTwo-year survival.
cOne-year survival.
All studies considered biopsy-proven acute rejection except from Junge et al. and Moench et al. in which this data is not specified.
Patient and graft survival: a3 months, b6 months, c1 year, d1, 3 and 5 years.
eAcute rejection at these interval times: 0-3 months, 3-6 months and 6-12 months;
ftreated acute rejection. gInfection episodes.
hData non-specified, but no difference between the two group was reported.
iStudy on pediatric patients.
j
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree