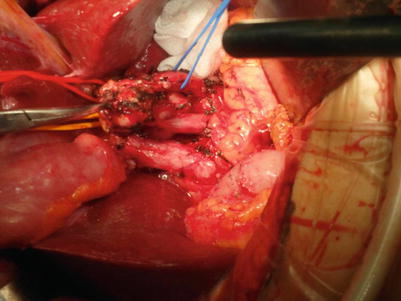
Fig. 21.1
Hjortsjo crook
Therefore, during the resection of segments 5 and 8, the cutting surface should not be overly close to the site where the right anterior branch separates from the portal vein; otherwise, the right posterior hepatic duct will be damaged and will cause difficulties during the reconstruction process.
21.2.2 Bile Ducts That Drain the Caudate Lobe
The caudate lobe is divided into the following three parts: (a) the spigelian lobe; which is located at the left side of the venous ligament and has one to two bile duct branches; (b) the paracaval portion, which is located in front of the vena cava and has one to two bile duct branches coming from the right posterior lobe; and (c) the caudate process, which is a small projection located between the vena cava and the right side of the portal vein into which one to two bile duct branches enter.
Because the site from where the caudate lobe bile duct issues is very close to the hilar confluence, hilar cholangiocarcinoma can readily invade this duct at early stages; accordingly, this duct should be resected.
The right hepatic artery typically runs behind the common hepatic duct, that is, beneath this duct after the confluence of the right and left hepatic ducts. This artery is often subjected to tumor invasion in cases of hilar cholangiocarcinoma (Fig. 21.2).
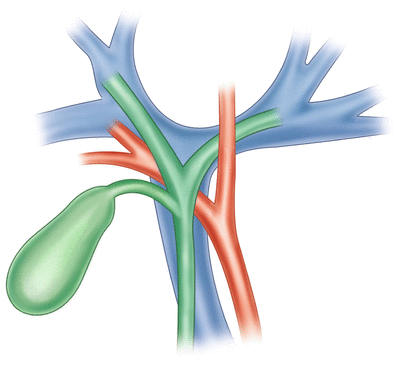

Fig. 21.2
The right hepatic artery typically runs behind the common hepatic duct
The right anterior branch of the right hepatic artery travels along the inner anterior side of the right branch of the portal vein, whereas the right posterior branch crosses the inner anterior side of the right branch of the portal vein and runs in Rouviere’s sulcus behind the gallbladder neck. These branches are easily freed and not particularly susceptible to tumor invasion; these characteristics facilitate the reconstruction of the right hepatic artery (Fig. 21.3).

Fig. 21.3
(a, b) The right anterior branch of the right hepatic artery travels along Rouviere’s sulcus behind the gallbladder neck
21.2.3 Hepatic Hilar Plate System
Glisson’s sheath and the connective tissue sheath surrounding the bile ducts and blood vessels below the liver fuse together to form the hilar plate system, which includes the hilar plate above the bile duct confluence, the cystic plate at the gallbladder fossa, the umbilical plate above the umbilical portion of the portal vein, and the Arantion plate covering the venous ligament (Fig. 21.4).
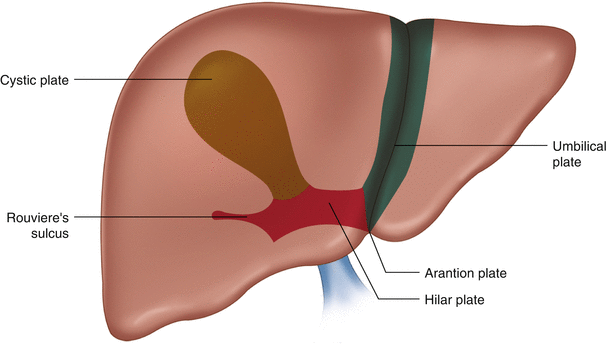

Fig. 21.4
Hepatic hilar plate system: a. cystic plate; b. hilar plate; c. umbilical plate; d. Arantion plate; e. Rouviere’s sulcus
In 1956, Hepp and Couinaud introduced the following approach: segment 4 of the liver was pulled upward to cut the bottom of Glisson’s sheath at its base, clearly revealing the hilar structure and indicating that vascular communication may only occur among 1 % of branches. However, in hilar cholangiocarcinoma, because the tumor readily invades adjacent hilar plate tissues and segment 1 of the liver, the hilar plate should not be dissected. Instead, en bloc resection of the bile duct confluence, the hilar plate, and the caudate lobe should be performed.
21.2.4 Lymphatic Drainage
The following two routes of lymphatic drainage are involved in hilar cholangiocarcinoma.
The left route travels through lymphatic vessels and lymph nodes (LN) along the cystic duct, the hepatic artery, and the anteromedial side of the portal vein to the celiac trunk; in other words, this route travels along LN #12a → 8 → 9 → 16.
The right route travels through lymphatic vessels and LN along the cystic duct and the anterolateral side of the portal vein, behind the pancreas, along the aorta, between the left side of the aorta and the vena cava, and below the left renal vein; in other words, this route travels along LN #12b → 13a → 16.
Therefore, regions that should be included in lymphadenectomy include the common hepatic artery, the celiac trunk, and behind the pancreatic head, although complete lymphadenectomy is difficult to accomplish.
21.3 Staging Systems
The seventh edition of the TNM (tumor, nodes, metastasis) staging criteria issued by the American Joint Committee on Cancer (AJCC) supplement the Bismuth classification system by considering tumor-related difficulties associated with the liver parenchyma, vascular structure, and invasion of the lymphatic system. Thus, this staging system provides information regarding tumor resectability based on pathological criteria.
☆ TNM classification (the AJCC stage 7th edition) | |||
|---|---|---|---|
Primary tumor (T) | |||
Tx | The primary tumor cannot be assessed | ||
T0 | No evidence of a primary tumor | ||
Tis | Carcinoma in situ | ||
T1 | The tumor is confined to the bile duct histologically | ||
T2a | The tumor invades the surrounding adipose tissue beyond the wall of the bile duct | ||
T2b | The tumor invades the adjacent hepatic parenchyma | ||
T3 | The tumor invades unilateral branches of the portal vein or hepatic artery | ||
T4 | The tumor invades the main portal vein or its branches bilaterally, the common hepatic artery, the second-order biliary radicals bilaterally, or the unilateral second-order biliary radicals with contralateral portal vein or hepatic vein involvement | ||
Regional lymph nodes (N) | |||
Nx | Regional lymph nodes cannot be assessed | ||
N0 | No regional lymph node metastasis | ||
N1 | Regional lymph node metastasis (cystic duct, common bile duct, hepatic artery, and portal vein) | ||
N2 | Metastasis to periaortic, pericaval, superior mesentery artery, and/or celiac artery nodes | ||
Distant metastasis (M) | |||
M0 | No distant metastasis | ||
M1 | Distant metastasis | ||
Stage group | |||
Stage 0 | Tis | N0 | M0 |
Stage I | T1 | N0 | M0 |
Stage II | T2a-T2b | N0 | M0 |
Stage IIIA | T3 | N0 | M0 |
Stage IIIB | T1-T3 | N1 | M0 |
Stage IVA | T4 | Any N | M0 |
Stage IVB | Any T | N2 | M0 |
Any T | Any N | M1 | |
21.3.1 Bismuth-Corlette Classification (Fig. 21.5)
Fig. 21.5
Bismuth-Corlette classification
This classification system was proposed by Bismuth and Corlette of France in 1975. In this system, tumors are categorized based on assessments of tumor location and the degree of longitudinal invasion along the biliary system; therefore, Bismuth-Corlette classification facilitates the clinical determination of the scope of surgical resection. This classification approach is simple and practical and has therefore become widely respected.
Type I: the tumor is below the confluence of the right and left hepatic ducts.
Type II: the tumor has reached the confluence of the right and left hepatic ducts.
Type IIIa and IIIb: the tumor has caused the embolization of the common hepatic duct and the left and/or right hepatic ducts.
Type IV: the tumor has invaded the confluence and the left and right hepatic ducts.
21.4 Preoperative Evaluation
21.4.1 Radiological Evaluation
Ultrasonography (US) is the preferred approach when jaundice is present because US can confirm biliary dilatation, locate obstruction sites, and exclude the possibility of stones [15]. Color Doppler is helpful for discovering portal vein and hepatic artery compression as well as tumor encapsulation [18].
Percutaneous transhepatic cholangiography (PTC) can display intrahepatic and hilar lesions.
Endoscopic retrograde cholangiopancreatography (ERCP) can reveal extrahepatic lesions. Although ERCP has limited application in cases of hilar cholangiocarcinoma, this technique is helpful for assessing drainage and stent placement.
Multidetector-row computed tomography (MDCT) can be used to not only examine tumor size, blockage levels, and hepatatrophia but also to evaluate resectability.
Magnetic resonance imaging (MRI) or magnetic resonance cholangiopancreatography (MRCP) can contribute to the assessment of biliary tumors, the determination of resectability, and the evaluation of the extent to which the tumor has invaded bile ducts, blood vessels, and the surrounding liver parenchyma [21].
21.4.2 Three-Dimensional (3D) Image Reconstruction
Hilar cholangiocarcinoma surgery now makes use of 3D imaging techniques [22–26]. With 3D imaging, the scope of patients’ tumors can be accurately determined, and 360° imaging of the biliary system can be performed to display each bile duct branch. This approach can be used to not only determine a tumor’s Bismuth type and whether a tumor has invaded the hepatic arteries and portal veins in its vicinity but also to assess the anatomical changes in the blood flow of hepatic vessels as well as the remnant liver volume. Thus, the use of 3D imaging can allow surgeries to be more precisely planned, which can reduce intraoperative blood loss and complications.
However, many unresolved difficulties remain.
21.4.3 Assessments of Hepatic Function
Hepatic function will be compromised in patients who suffer from obstructive jaundice. Hepatic function can be evaluated using the Child-Pugh scoring system, the model for end-stage liver disease (MELD), and the indocyanine green (ICG) clearance test.
Computed tomography (CT) is used to assess remnant liver volume, and CT and MRI are used to measure the extent of steatosis in patients’ livers. Typically, the remnant volume of a normal liver should be >25 %; in patients with hepatic dysfunction, remnant liver volume should be >40 % [27].
The regenerative capacity of the liver has been investigated using embolism of the right branch of the portal vein to induce left liver hyperplasia; this approach has shown that non-embolized hepatic tissues can typically increase in size by 30 % in patients with normal regenerative capacities.
21.4.4 Preoperative Assessment
Hilar cholangiocarcinomas can exhibit exophytic, infiltrating, polypoid, or mixed types of growth. Periductal-infiltrating tumors account for 70 % of hilar cholangiocarcinomas [28, 29]. Radiological evaluation is first performed to determine a tumor’s scope, hepatic parenchymal invasion status, vascular invasion status, the extent to which liver lobes have atrophied, the number of metastatic lesions, and the extent to which LN metastasis and distant metastasis have occurred.
Cases involving any of the following conditions are typically unresectable [20, 30]: ① extensive invasion at the confluence of the left and right hepatic ducts; ② invasion of the main trunk of the portal vein; ③ concurrent invasion of the left and right branches of the portal vein; ④ invasion of the left or right portal vein, combined with extensive invasion of the bile ducts on the opposite side; ⑤ extensive LN metastasis; and ⑥ distant metastasis.
However, surgical evaluation is ultimately necessary to determine resectability.
21.4.5 Laparoscopic Assessment
CT and MRI can typically confirm the extent of portal vein involvement in cases of hilar cholangiocarcinoma. However, metastatic lesions in the liver, the greater omentum, and the peritoneum are difficult to discover. Therefore, in recent years, many researchers have recommended performing an initial laparoscopic procedure to detect small metastatic lesions and thereby avoid unnecessary laparotomies [31].
Furthermore, laparoscopic US (LUS) can discover intrahepatic metastatic lesions and local vascular invasion that cannot be detected through imaging examinations; thus, LUS can contribute to the determination of resectability.
21.5 Preoperative Preparation
Hilar cholangiocarcinoma usually manifests as jaundice and leads to liver damage. In addition, these tumors frequently invade portal veins, hepatic arteries, and peripheral hepatic parenchyma; as a result, resection is difficult, with operative mortality rates of up to 20 % and complication rates of up to 67 %. Therefore, accurate preoperative evaluation and careful preparation are extremely important [32–34].
21.5.1 Preoperative Biliary Drainage (BD)
The objectives of BD are as follows: ① reduce bilirubin levels, ② treat biliary tract infections, and ③ allow for explicit radiographic confirmation of the extent to which a tumor has spread.
The risks of BD are as follows: ① implantation of approximately 5 % of tumors, ② infection, and ③ bleeding.
The modes of BD are as follows: ① endoscopic nasobiliary drainage (ENBD) and ② percutaneous transhepatic biliary drainage (PTBD). Typically, unilateral drainage is sufficient, and drainage should continue for 4–6 weeks until a patient’s total bilirubin level has decreased to 2–3 times the upper limit of the normal range [35]. Because BD has risks and most jaundice patients can tolerate extended hepatectomy [36], many medical centers do not recommend preoperative BD.
21.5.2 Preoperative Portal Vein Embolization (PVE)
The purpose of PVE is to induce the regeneration of the future liver remnant (FLR) and thereby reduce postoperative liver failure and death.
Indications:
① patients with liver remnants of <40 % and ② patients who will undergo extended hepatectomy, particularly patients with possible revascularization [37].
Methods:
BD is first performed for 4–6 weeks; when the total bilirubin has decreased to 50 u, PVE is performed. Hepatectomy can be performed 2–3 weeks after PVE.
21.5.3 Two-Stage Resection Approach
A two-stage resection approach may be utilized for patients with indications for PVE. The first stage involves the surgical transection of the liver parenchyma and the concurrent ligation of bile duct and portal vein branches without the transection of hepatic arteries or hepatic veins. After 1 week, when the liver remnant exhibits hyperplasia, the second stage of the liver resection can be performed [38].
The advantage of this method is that this approach can significantly shorten preoperative preparation time.
21.5.4 Preoperative Laparoscopic Staging
An initial laparoscopic exploration is recommended for hilar cholangiocarcinoma patients whose tumors have been confirmed to be resectable by preoperative CT, MRI, or other approaches and who will undergo PVE. If this laparoscopic examination detects peritoneal and/or omental metastatic lesions or extensive small intrahepatic metastatic lesions, then surgical resection should be abandoned, and palliative treatments, such as the placement of biliary stents, should be performed to shorten the patient’s hospital stay. LUS can be performed to further determine a patient’s situation with respect to intrahepatic metastatic lesions and portal vein invasion. Thus, laparoscopic staging should be performed to avoid unnecessary laparotomies.
21.6 Consideration of the Surgical Procedure for Hilar Cholangiocarcinoma
Numerous clinical reports have indicated that only radical resection with histologically negative surgical margins (R0) is required to achieve radical resection of cholangiocarcinomas [39, 40]. Therefore, there is a broad spectrum of hilar cholangiocarcinoma surgeries, which range from local hilar resection or limited hepatectomy to mesohepatectomy, extended left and right hepatectomy, and caudate lobe resection [41, 42]. To achieve R0 resection, for certain patients with advanced-stage hilar cholangiocarcinoma or with relatively widespread invasion, an extended hemihepatectomy with vascular resection and reconstruction [43, 44] or a comprehensive hepatopancreatoduodenectomy (HPD) [43, 44] should be performed. Thus, the current surgical options for hilar cholangiocarcinoma include the following approaches:
1.
Mesohepatectomy – suitable for Bismuth types I, II, and III
2.
Extended right hemihepatectomy – suitable for patients with Bismuth type IIIa or IV tumors and invasion of the right branch of the portal vein
3.
Extended left hemihepatectomy – suitable for patients with Bismuth type IIIb or IV tumors and invasion of the left branch of the portal vein
4.
Extended right hemihepatectomy with portal vein resection – suitable for patients with Bismuth type III or IV tumors and invasion of the main trunk or bifurcation of the portal vein
5.
Combined hepatic artery resection and reconstruction – suitable for patients with hepatic artery invasion
6.
Combined HPD – suitable for patients with lower common bile duct and pancreatic head invasion
21.7 Mesohepatectomy (Resection of Segments IVb, V, and I)
1.
Separation is performed at the superior edge of the pancreas and the left edge of the hepatoduodenal ligament. Lymphadenectomy is performed upward along the hepatic artery and portal vein to skeletonize this region (Fig. 21.6).
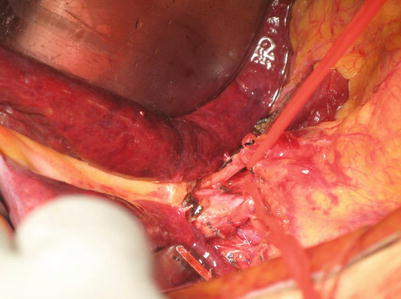

Fig. 21.6
Separation is performed at the superior edge of the pancreas and the left edge of the hepatoduodenal ligament
2.
Separation is performed along the superior margin of the pancreas to the right edge of the hepatoduodenal ligament. The common bile duct is transected upward to free the extrahepatic bile duct and the posterior wall of the gallbladder until the posterior side of the hilar tumor is reached. Surgeons then confirm that the main trunk and bifurcation of the portal vein have not been invaded. If the right hepatic artery has been invaded by the tumor, then this artery can be transected (Fig. 21.7).