Equipment
Fluoroscopy table
Rigid and flexible cystoscopes
Semirigid ureteroscope
Flexible ureteroscope
Disposable
Hydrophilic guidewires
Holmium laser
5-F ureteral catheter
Biopsy forceps
Ureteroscopic Bugbee electrode
JJ-ureteral stent
Contrast solution
Surgical Technique
Both ureteral stricture and upper tract urothelial carcinoma cases begin with a thorough cystoscopic evaluation of the lower urinary tract using a 22-F rigid cystoscope. Use of both a 30° and a 70° lens provides optimal visualization of the entire bladder urothelium and ureteral orifices. If lesions are identified within the bladder at time of cystoscopy, they are biopsied and treated after treatment of the upper tracts. Alternatively, a flexible cystoscope can be used in patients with a urostomy and urinary diversion. In these patients, due to the unpredictable location of the ureterointestinal anastomoses, intravenous indigo carmine (1 ampule) is given at the beginning of the case to aid in localization of the ureteroenteric anastomoses. Regretfully, in some cases the ureter cannot be accessed in a retrograde fashion and the patient must then undergo a percutaneous antegrade approach.
Regardless of the indications for ureteroscopy, a retrograde pyelogram is performed to assist in defining the patient’s anatomy and identifying any anatomical abnormalities (Fig. 28.1). A 5-F cone tip catheter is placed through the cystoscope and flushed prior to cannulation of the ureteral orifice. This removes any potential confusion created by air bubbles appearing as filling defects during the retrograde pyelogram. A scout film is taken, the cone tip catheter is inserted into the ureteral orifice, contrast is injected, and fluoroscopic images are obtained. In the case of a ureteral stricture, this helps define location and length. With upper tract transitional cell carcinoma, tumors typically are identified as filling defects. In both situations, the retrograde pyelogram provides a “road map” for the remainder of the case.
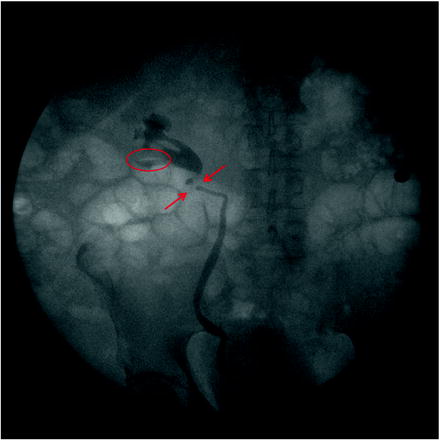

Fig. 28.1
A retrograde is performed at the beginning of the case. A filling defect is clearly identified within the lower pole and proximal ureter. The retrograde pyelogram then serves as a “road map” for the remainder of the procedure
Stricture
Following retrograde pyelography, under fluoroscopic guidance, a 0.38 Glidewire (Boston Scientific, Natick, MA) is placed through the cone tip catheter beyond the stricture and coiled within the renal pelvis. For tight strictures, this may need to be accomplished with glidewires in conjunction with angle tipped ureteral catheters. In rare cases when a glidewire cannot bypass a stricture fluoroscopically, attempts can be made under direct endoscopic visualization with a semirigid ureteroscope placed distal to the stricture. If despite all efforts a wire cannot transverse the stricture, the procedure is aborted, and the patient is scheduled for subsequent open repair.
Once the wire is coiled within the renal pelvis, the ureteral catheter and cystoscope are removed, leaving the wire behind. This wire is then exchanged for a stiffer wire, such as a Sensor (Boston Scientific, Natick, MA) or Amplatz (Cook Medical, Bloomington, IN) super stiff wire, which will act as the working wire. In all cases, we prefer to place an additional wire to serve as a safety wire to prevent loss of access. This is achieved with an 8/10-F coaxial dilator, which acts to both dilate the intramural ureter and to introduce the second wire. The 8/10-F coaxial dilator is placed over the original guidewire and the 8-F portion is removed, leaving the 10-F sheath behind. Through this, our safety wire is passed and coiled within the renal pelvis. Fluoroscopy is used throughout this process taking diligent care to minimize exposure to the patient and operative team. Our designated safety wire is then secured to the drapes on the contralateral side to minimize risks of inadvertent removal during the case.
Once access is obtained, the ureteroscopic equipment is assembled. In order to minimize intrarenal pressure and subsequent pyelovenous backflow during ureteroscopy, we prefer the use of a hand pump irrigator. Additionally, a ureteral access sheath may be used for mid and proximal ureteral strictures to further decrease intrarenal pressures [8]. Irrigation fluid is typically normal saline. However, in our experience, using water for nearly all ureteroscopic cases has been a safe alternative with improved visualization. The ureteroscope is passed over the working wire to the level of the stricture under fluoroscopic guidance. The wire is then removed, leaving the ureteroscope and the safety wire behind. Sometimes traversing the intramural ureter with the ureteroscope is difficult. Using a balloon dilator to dilate the intramural ureter is an option, although this should be done only when necessary.
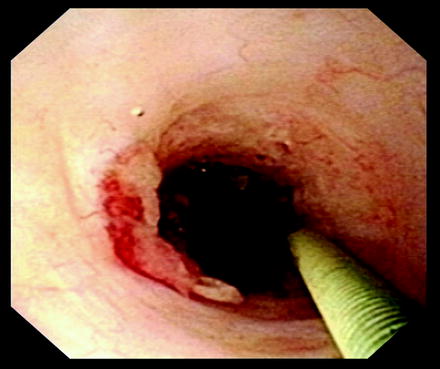
The length, location, degree of stenosis, and the presence of pulsations are assessed fluoroscopically and endoscopically. Prior to treatment, a biopsy should be performed in patients with a recurrent stricture, a suspicious appearing stricture, a stricture of unclear origin, or a history of urothelial malignancy. Endoureterotomy is then performed under direct vision using an end-firing pulsed holmium laser fiber at a setting of 1 J and 10 Hz (Fig. 28.2). A full thickness incision is made into the periureteral fat. The incision is started proximally and carried distally, taking care to incise the ureter for the entire length of the stricture including normal ureteral margins. When making the incision, it is important to consider the vascular supply to the ureter. Incisions are made laterally for strictures in the proximal and midureter and medially for strictures in the distal ureter to avoid vascular injuries. Following laser incision, the ureteroscope is positioned distal to the stricture and a retrograde ureteropyelogram is performed through the scope. Extravasation of contrast fluoroscopically verifies full thickness incision. The remainder of the ureter is then examined prior to removal of the ureteroscope to confirm there are no additional sites of stricture.
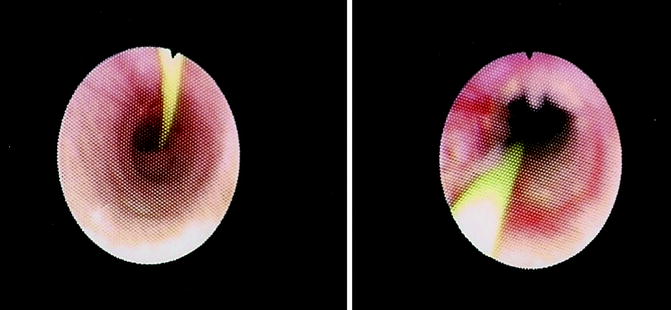

Fig. 28.2
A ureteral stricture is seen in these images, pre- and postendoureterotomy
A ureteral stent is then placed over safety wire under fluoroscopic guidance. An endopyelotomy stent can also be used in this situation for temporary internal drainage. Endopyelotomy stents have the advantage of having a larger diameter (10–18 F) at the incised stricture, which in theory may result in higher success rates. However, multiple studies have not demonstrated an advantage when compared to standard stents [9–11]. After full thickness incision of the ureter and stenting, the ureteral wall reforms completely within 2–6 weeks [12]. We therefore typically leave the stent in place for 4–6 weeks.
Tumor
In contrast to the practice of gaining access for ureteral strictures, it is important that no wires are passed prior to complete inspection of the ureter and calyceal system in the evaluation and treatment of upper tract urothelial carcinoma. Any irritation of the urothelium from iatrogenic wire trauma may cause bleeding or mimic a urothelial tumor. Initially, a semirigid ureteroscope is passed into the midureter and complete inspection is performed. Once this has occurred, a guidewire is passed through the semirigid ureteroscope to the midureter but not beyond the previously inspected area. The semirigid ureteroscope is removed and over the wire the flexible ureteroscope is passed. The wire is removed and the flexible ureteroscope is advanced into the renal pelvis under direct visualization. A thorough inspection of the kidney and ureter is performed, using fluoroscopy as an aid in accessing the entire calyceal system (Fig. 28.3).
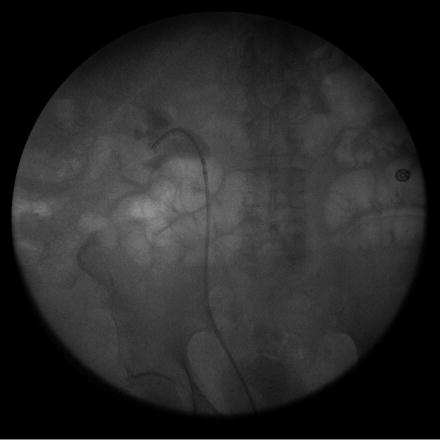

Fig. 28.3
The flexible ureteroscope is employed to perform renoscopy. Here the scope is within the midpole. Using the retrograde pyelogram, the surgeon can ensure complete renoscopy is performed
In general, a distal ureteral pathology can be accessed with a semirigid ureteroscope while a lesion in the mid/proximal ureter or renal collecting system is best approached with a flexible ureteroscope (Fig. 28.4). As with strictures, if tumors are identified in the mid or proximal ureter or kidney, a ureteral access sheath can be used to improve visualization and reduce intrarenal pressure [8]. Once tumors are endoscopically identified, samples are obtained via aspiration adjacent to any visible tumors. Secondly, a barbatoge specimen using normal saline is obtained. For visible tumors, biopsies are taken using the Pirhana (Boston Scientific, Natick, MA) or BIGopsy (Cook Medical, Bloomington, IN) biopsy forceps. Since the BIGopsy forceps need to be back loaded onto the scope, a ureteral access sheath must be used when using this instrument. Alternatively, in an effort to garner more tissue, large papillary tumors can be snared with a wire basket. For flat lesions and those in the ureter, a brush biopsy can be performed. Following biopsies, fluid should again be aspirated and a barbatoge specimen should be acquired. In all cases, multiple samples should be obtained, and all should be sent to the cytopathology laboratory for analysis. Obtaining specimens as stated above, will improve diagnostic yield. In studies by Kelley and Tawfiek, when these techniques are employed, the ability to grade tumors increases from 42.9 to 97.2% [13, 14].