Laparoscopic Surgery: Introduction
Laparoscopy plays a prominent role in urology. Current residents finish their training with considerable exposure to the techniques, and a plethora of courses educate physicians already in practice. Alternatives to standard laparoscopy, including hand assistance and robotic assistance, further enhance capabilities.
Physiology of Laparoscopy
Laparoscopy with pneumoperitoneum exposes the patient to physiologic challenges that differ from that of open surgery, but can be met successfully with proper preparation and awareness (Ost et al, 2005).
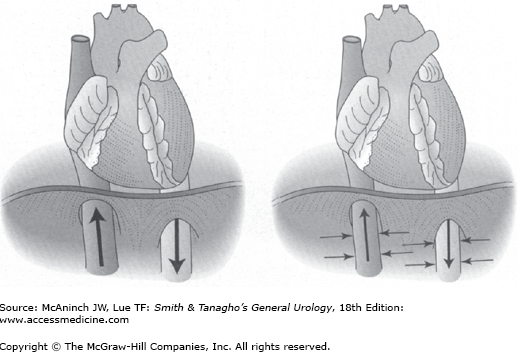
As intra-abdominal pressure increases with pneumoperitoneum, the systemic vascular resistance increases and venous return decreases. A small increase in intra-abdominal pressure augments venous return and cardiac output. As intra-abdominal pressure rises, the increase in resistance exceeds the increase in pressure, and venous return and cardiac output fall (Figure 9–1). This transition point occurs at a lower intra-abdominal pressure in the hypovolemic compared with the normovolemic state. Given normovolemia, an intra-abdominal pressure of 15 mm Hg is associated with tolerable reduction of cardiac output.
The absorption of insufflated carbon dioxide (CO2) has direct cardio-inhibitory effects, but CO2 also stimulates the sympathetic nervous system. If acidosis develops then there are parasympathetic effects as well. Moderate hypercapnia (excess CO2 in blood) produces an increase in cardiac output and blood pressure and a decrease in systemic vascular resistance, which counteract the effect of intra-abdominal pressure.
Overall, an intra-abdominal pressure of 15 mm Hg and moderate hypercapnia in healthy patients produce a hyperdynamic state (increased central venous pressure, systemic vascular resistance, heart rate, and blood pressure) without significant alteration of cardiac output (Junghans et al, 2005).
The cardiovascular complications of laparoscopy include tension pneumoperitoneum, cardiac dysrhythmias, fluid overload, and venous thrombosis.
When the intra-abdominal pressure is excessive, usually >40 mm Hg, the overwhelming increase of vascular resistance can produce “tension pneumoperitoneum.” Venous return, cardiac output, and blood pressure drop precipitously. Volume status must be optimized to prevent tension pneumoperitoneum at lower pressures. In general, the intra-abdominal pressure should be kept below 15–20 mm Hg. The response to “tension pneumoperitoneum” should be immediate desufflation.
Tachycardia and ventricular extrasystoles due to hypercapnia are usually benign, but fatal dysrhythmias can occur with very high arterial partial pressure of CO2 (PaCO2). Vagal stimulation by peritoneal distention can produce bradydysrhythmias (Valentin et al, 2004).
Because insensible fluid losses and urine output are less during laparoscopy than during open surgery, after optimizing volume status before insufflation intraoperative fluid administration should be limited to appropriate replacement for blood loss plus a maintenance rate of 5 mL/kg/h to avoid volume overload.
Since increased abdominal pressure during laparoscopy restricts lower extremity venous return, sequential compression devices may be the best choice for venous thrombosis prophylaxis during major laparoscopic procedures (Montgomery and Wolf, 2005).
Increased intra-abdominal volume elevates the diaphragm, which reduces lung capacity and compliance. Insufflated CO2 diffuses into the bloodstream. The amount of CO2 absorbed during intraperitoneal CO2 laparoscopy at typical pressures is equivalent to adding 5–25% to the body’s baseline production of CO2. Subcutaneous emphysema, elevated intra-abdominal pressure, extraperitoneal insufflation, and increased duration of insufflation all increase the rate of CO2 absorption.
The pulmonary, acid–base, and insufflant-related complications of laparoscopy include hypercapnia, acidosis, extraperitoneal gas collections, and venous gas embolism (VGE).
Moderate hypercapnia is stimulatory overall, but if PaCO2 exceeds 60 mm Hg, then potentially lethal cardiodepressive effects predominate. Increasing ventilation rate and tidal volume adequately eliminates excess CO2 in most patients. PaCO2 is estimated intraoperatively by the partial pressure of end-tidal CO2 (P[et]CO2) measured with capnometry, which generally is 3–5 mm Hg lower than PaCO2 during general anesthesia. During prolonged operations or in patients with pulmonary disease, the gradient may widen unpredictably, and arterial blood gases should be obtained for accurate monitoring (Kim, 2008).
Absorption of insufflated CO2 causes a mild respiratory acidosis. With gas insufflation pressures >20 mm Hg, a metabolic acidosis also can develop, likely related to retained acids from decreased urine output.
Gases insufflated into the peritoneal cavity may leak into several extraperitoneal spaces. Subcutaneous emphysema is the most common site of extraperitoneal gas. Although generally innocuous, it increases the risk of hypercapnia (Saggar et al, 2008). Pneumopericardium, pneumomediastinum, and pneumothorax can inhibit cardiac filling and/or lung excursion. A CO2 pneumothorax will usually resolve spontaneously, but thoracostomy should be performed for a symptomatic pneumothorax (Msezane et al, 2007).
VGE is the passage of gas bubbles through the venous system into the heart and pulmonary circulation (Min et al, 2007). When clinically significant, right heart outflow is impeded, producing hypoxemia, hypercapnia, and depressed cardiac output. Many VGEs during laparoscopy have been fatal. VGE is indicated by hypoxemia, pulmonary edema, increased airway pressure, hypotension, jugular venous distention, facial plethora, dysrhythmias, and a mill-wheel murmur. The capnometer will register a sudden fall in P(et)CO2 if the CO2 embolus is large. Swift response is required, including immediate desufflation, rapid ventilation with 100% oxygen, steep head-down tilt with the right side up, and general resuscitative maneuvers.
Selection of Laparoscopic Approach
There are a number of alternative approaches to laparoscopic surgery. Access can be transperitoneal or retroperitoneal. Options for surgical manipulation include standard laparoscopy, hand assistance, and robotic assistance. Additional modifications are being developed, such as microlaparoscopy, laparoendoscopic single-site surgery (LESS), and natural orifice translumenal endoscopic surgery (NOTES) (Kommu et al, 2009; Raman et al, 2008). Only the most common alternatives are described here.
The transperitoneal route provides a capacious working space and allows direct visualization of familiar intraperitoneal anatomy. It is the most common route for most procedures. Transperitoneal access may be difficult, however, in patients who have had extensive abdominal surgery. The retroperitoneal approach allows easier and more rapid access to the retroperitoneal structures and avoidance of intra-abdominal organs and adhesions. The disadvantage of the retroperitoneal technique is the limited working space. Comparisons of the two routes for a variety of procedures have shown no consistent difference in operative times, cost, length of stay, or postoperative convalescence (Desai et al, 2005). The approach therefore is dictated by the familiarity of the surgeon and the patient’s condition.
Hand-assisted laparoscopic surgery (HALS) entails the insertion of a hand through a 7–9-cm incision into the laparoscopic field, while maintaining pneumoperitoneum with a hand-assistance device that employs a compressive mechanism to affix to the abdomen and prevent leakage of pneumoperitoneum around the intra-abdominal hand (Figure 9–2). The intra-abdominal hand is used for dissection, tissue identification, retraction, and control of injuries. The benefits of HALS include shorter operative times than for similar standard laparoscopic transperitoneal procedures in many series, ease of learning by inexperienced surgeons, and enhanced ability to manage difficult surgical situations. Additionally, conversion from standard laparoscopy to hand assistance is an alternative to conversion to open surgery. Disadvantages of HALS include problems with the devices (gas leakage or interference with port placement), physical strain on the hand, interference of the hand in the operative field, and the creation of a larger incision (and likely more wound complications) than for a standard laparoscopic procedure (Wolf, 2005). Numerous comparative studies between standard and hand-assisted laparoscopy show generally similar convalescence (Silberstein and Parsons, 2009).
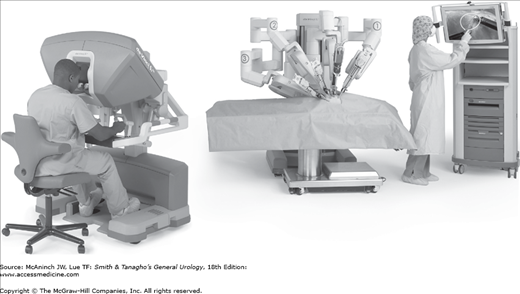
The robot for laparoscopic surgery (da Vinci Surgical System; Intuitive Surgical, Sunnyvale, CA) has a three-dimensional camera and arms that are directed by the surgeon seated at the control console (Figure 9–3). It has achieved great popularity for application to radical prostatectomy and cystectomy, and it is being increasingly used for renal surgery as well. The advantages of robotic assistance include easier transference of open surgical skills to laparoscopic surgery, instrument tips with multiple degrees of freedom, and better ergonomics than standard laparoscopy. Disadvantages include the cost of the system and disposables and the need for a trained assistant at the bedside (Nelson, 2007).
Laparoscopic Instrumentation and Basic Techniques
Although any operation in a patient with obesity, previous abdominal surgery, or abnormal anatomy is difficult, laparoscopy is relatively more challenged by these factors than is open surgery. In addition, open surgery may be favored physiologically over laparoscopy in patients with severe pulmonary disease or congestive heart failure. The patient being offered laparoscopy should be fully informed of the risks and benefits, most appropriately in the context of a comparison with the spectrum of risks and benefits of the corresponding open surgery. Inform the patient of the surgeon’s experience with the particular laparoscopic procedure, and advise that conversion to open surgery may be required. For transperitoneal laparoscopic surgery without intended bowel resection, patient preparation with a clear liquid diet and oral magnesium citrate on the preoperative day is adequate. For retroperitoneoscopic surgery, bowel preparation is not necessary.
The primary laparoscopic cart (see later) is positioned opposite the surgeon. For an upper abdominal or retroperitoneal procedure, a secondary monitor for nursing staff or assistants placed on the opposite side of the patient is useful. Operating rooms with equipment booms from the ceiling minimize clutter and reduce setup time. After induction of anesthesia and endotracheal intubation, insert a urethral catheter and orogastric tube. For pelvic surgery, position the patient supine (or in some cases, dorsal lithotomy), with the chest securely taped to allow steep Trendelenburg tilt of the table. For transperitoneal procedures into the retroperitoneum, place the patient in partial flank position (45°) without flexion of the table. Direct retroperitoneoscopic procedures benefit from full flank position with table flexion.
Initial access is commonly obtained with the closed (Veress needle) or open (Hasson cannula) techniques. Either is acceptable, but if the Veress needle is the first choice then the open technique should be learned as well, since sometimes the former is contraindicated or fails.
The Veress needle has a spring-loaded stylet that retracts back only under pressure from firm tissue (ie, fascia) to expose the sharp cannula; once the tip is free in the intraperitoneal space, the stylet springs forward and protects against visceral injury. The needle is usually placed at the location of the first port, although the site should be moved away from any prior incisions (extensive abdominal surgery or suspected dense adhesions are a relative contraindication for Veress needle use). The needle is inserted almost perpendicular to the abdominal wall, tilting slightly away from the large midline vessels. An exception is when the needle is being placed at the umbilicus in thin individuals, in which case it needs to be angled more acutely.
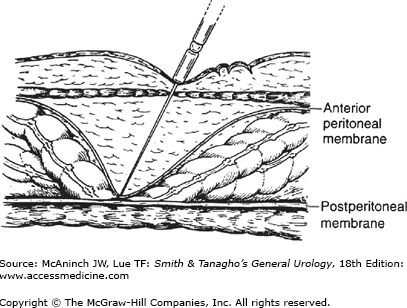
After inserting the needle, attach a 10-cc syringe half-filled with saline and aspirate back. There should be no aspirated gas or liquid. Next, inject saline through the needle and attempt to aspirate. Fluid should flow in easily and not return. Finally, the saline in the hub of the Veress needle should drop rapidly into the abdomen. These maneuvers assess for the possibility that the needle tip is in a luminal structure (bowel, blood vessel, etc), but all will be “normal” if the needle is preperitoneal—which is the most common erroneous placement (Figure 9–4). This possibility is assessed by the final test, the “opening pressure” as gas is insufflated. The pressure should not rise above 8 mm Hg within the first one-half liter of gas, or if it does, it should only be momentary and quickly correctable by a twist, slight withdrawal, and tilting up of the needle (which will free the needle tip from omental or mesenteric fat). If these conditions are met, then continue insufflation, but if not, then disconnect the insufflation tubing, allow the gas to escape, and withdraw the needle.
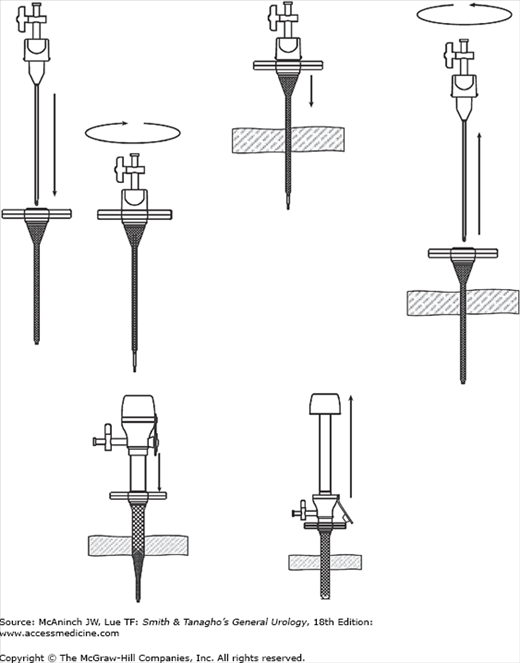
The Hasson technique is placement of a laparoscopic port through a small incision under direct vision. Make a 1.5–3-cm incision down to and through the peritoneal membrane. Place stay sutures and then insert the Hasson port (10/12 cannula containing a blunt obturator, with a conical adjustable sleeve, Figure 9–5). Tie the sutures to the arms of the device to secure the conical sleeve within the fascia. An alternative port has a retaining balloon that inflates intra-abdominally and is held up tight against the fascia by an adjustable retaining ring (Figure 9–6). Another alternative is to make a smaller incision in the fascia and peritoneal membrane and insert a blunt port that dilates rather than cuts the fascia (see later). Finally, a visualizing trocar (see later) can be used without prior insufflation.
The general scheme of port placement is to surround the site with the necessary number of instruments placed widely enough apart that they do not “sword fight” in the abdomen and with the laparoscope situated so that a good visual angle can be attained.
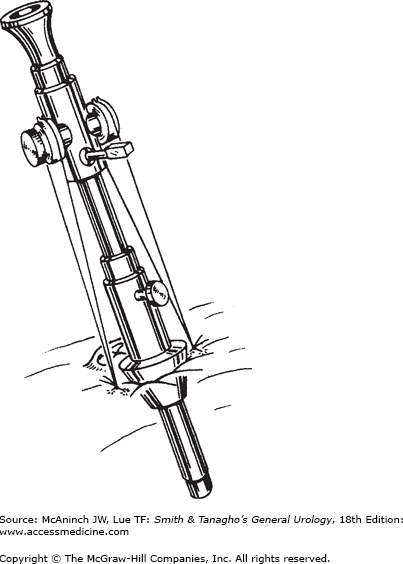
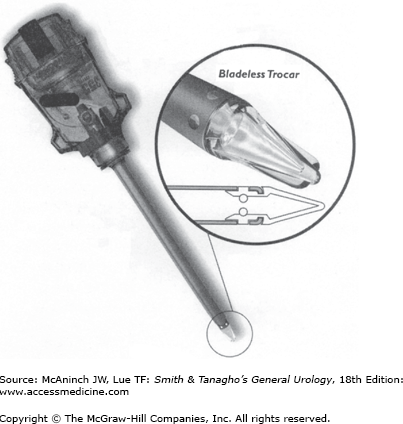
Standard port sizes range from 5 to 12 mm. “Needlescopic” ports (3 mm) as well as extra-wide ports (18 and 30 mm) are available for specialized use. Ports can be disposable, reusable, or “reposable.” The third contains both reusable and disposable components. The trocars inside the ports can be cutting or noncutting. Disposable sharp trocars are shielded (Figure 9–7). Some reusable trocars have nonshielded sharp tips. The Hasson-type ports employ blunt tips. There are ports with clear plastic tips (Figure 9–8) that allow visualization with the laparoscope as the port is being inserted. The Step system (AutoSuture, Norwalk, CT; Figure 9–9) employs an expandable sheath that is inserted with a Veress needle. The needle is removed and a dilating trocar is used to insert the port. This and other ports that dilate or screw into the fascia make a smaller fascial defect and pose less risk of injury than sharp-tipped ports.
If the Veress needle has been used for abdominal entry, the next step is to place the first port, which is usually for the laparoscope and inserted at the site of Veress needle placement. To avoid inadvertent removal of the port during the procedure, anchor it to the skin with suture. Alternatively, special sheaths can secure the port. Subsequent ports are placed under visual control through the laparoscope.
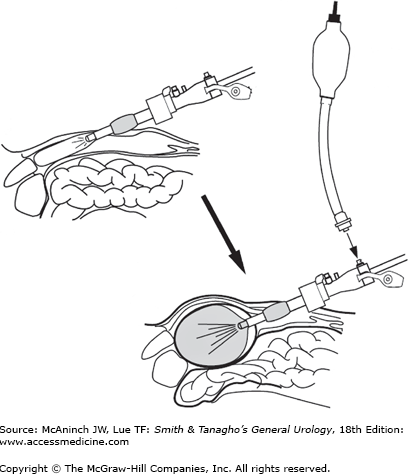
For flank retroperitoneoscopic surgery, make a 2-cm incision near the tip of the 12th rib down through the lumbodorsal fascia under direct vision. Explore the retroperitoneal space with a finger, and then dilate the space with a balloon (commercially available, Figure 9–10, or self-made). Insert a self-retaining port at this location, and place additional ports as needed. For pelvic extraperitoneal surgery, direct the balloon through an infraumbilical incision toward the pubic bone and dilate the preperitoneal space.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree