Fig. 19.1
Graph showing proportion of benign and malignant resections from a prospective Laparoscopic Liver Resection registry at Southampton General Hospital
19.2 Benign Liver Lesions
A large percentage of benign liver lesions are identified incidentally during an imaging procedure performed for unrelated condition. In addition, with advances in imaging technology, previously undetected lesions are now identified. Cherqui D et al. have reported a sixfold increase in diagnosis of FNH over an 8-year period from 1985 to 1992 [27]. In order to recommend appropriate management, the surgeon must be aware of the different features of benign tumors, their natural history, and the diagnostic accuracy of various imaging techniques. Suspicious lesions represent a clinical management dilemma for the risk of missing a premalignant or malignant lesion or subjecting the patient to an unnecessary surgery [28–31]. In the absence of chronic liver disease or malignancy, most of these lesions are benign and will require no further treatment. A systematic approach for the work-up of a benign liver lesion is recommended. The importance of history taking and physical examination cannot be underestimated. The vast majority of benign cystic lesions of the liver do not require treatment. Rarer forms of these lesions may have an element of diagnostic uncertainty. The diagnostic dilemma is more pronounced in solid lesions as compared to cystic lesions.
19.2.1 Principles of Management
Principles of management of benign liver lesions are:
1.
Confirmation that the lesion is benign
2.
Determining whether the lesion requires surgical treatment
3.
If not for surgical resection, to determine the optimal follow-up of these lesions, if needed
19.2.2 Indications for Surgery
Indications for surgery in benign liver lesions are:
1.
Preoperative diagnosis of adenoma or cystadenoma.
2.
Symptoms affecting quality of life. It is important to correlate that patients’ symptoms are indeed related to the presence of the liver lesion. This may mandate further investigations, e.g., gastroscopy to evaluate upper abdominal symptoms. Cherny CK et al. in a study of 155 benign liver tumors concluded that symptomatic patients with a small focal nodular hyperplasia (FNH) or hemangioma can be observed because their symptoms are unlikely to be related to the liver tumor and percutaneous needle biopsy rarely changed management [32].
3.
Diagnostic uncertainty. Figures 19.2 and 19.3 show a scan of a patient who presented with abdominal discomfort, and there was diagnostic uncertainty on imaging.
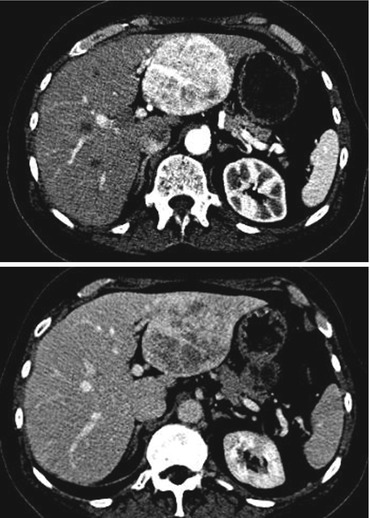

Figs. 19.2 and 19.3
Computerized tomography (CT) scan images of a 65-year-old female patient with abdominal pain and a 7.5 cm arterial avid lesion with washout. The radiological features were equivocal, and based on symptoms with diagnostic dilemma, surgical removal was recommended by the multidisciplinary team. She underwent a laparoscopic modified left hemihepatectomy, and postoperative histology confirmed this to be an angiomyolipoma of the liver
4.
Complications of bleeding or rupture.
Weimann et al. [28] in a study involving observation of asymptomatic 157 benign liver tumors (103 hemangiomas and 54 with FNH) found a radiologic diagnostic accuracy of >90 % and no tumor-related rupture or malignant transformation during a median follow-up of 32 months. Also follow-up imaging showed that in 80 % of these patients, the size remained stable, and in 7 % of patients, the tumor regressed in size. They concluded that in asymptomatic benign liver tumors, as the size remains stable for many years, indications for surgery should be carefully evaluated. In the same series, 173 patients underwent surgery for benign liver tumors with a mortality of 0.6 %. In a study spanning over a 7-year period and involving 51 women of <50 years of age with a preoperative diagnosis of either FNH or hepatic adenoma (HA), 6 % of patients had a malignancy on histology [34].
19.2.3 Common Benign Liver Lesions
Hemangiomas, focal nodular hyperplasia (FNH), hepatic adenomas (HA), hepatic cysts, and hepatobiliary cystadenomas are common benign liver lesions, and we shall discuss them briefly [35]. Liver resection is also indicated in hepatolithiasis complicated with recurrent cholangitis, biliary strictures, liver atrophy, or secondary biliary cirrhosis [36].
19.2.3.1 Hemangioma
Hemangiomas are the most common benign liver tumors with an incidence of up to 20 % of the general population. These lesions have no malignant potential. Symptomatic hemangiomas often tend to be large, varying from 5 to 20 cm in diameter. Hemangiomas tend to grow by expansion and push liver tissue away as they grow. This creates a fibrolamellar plane of tissue that defines the border between a hemangioma and surrounding normal liver. Indications for resection have been a development of symptoms, consumptive coagulopathy, or Kasabach-Merritt syndrome [37]. Enucleation preserves normal parenchyma, limits blood loss, and has fewer complications than a segmental resection [38–40].
19.2.3.2 Focal Nodular Hyperplasia
Focal nodular hyperplasias (FNHs) are solid benign tumor of the liver and occur in 3 % of the population. FNH has no malignant potential and usually remains stable or decrease in size over time. Less than 20 % of patients develop symptoms or complications, and if so, may be resected [37].
19.2.3.3 Hepatic Adenoma
Hepatic adenomas (HAs) have an epithelial origin and are occasionally associated with the use of estrogen (contraceptive pills) and steroids. They are more frequently found as solitary lesions but sometimes can be multiple. The arguments in favor of the conservative approach are that discontinuation of oral contraceptive pills may induce HA regression; it is not always certain that abdominal symptoms are related to the HA; if bleeding occurs, this can be managed conservatively, and surgical resection has 10–27 % morbidity and up to 3 % mortality. Resolution of HA following cessation of oral contraceptive pills was uncommon in a Mayo Clinic series [41]. The arguments in favor of the surgical approach are difficulty of preoperative imaging in differentiating HA from hepatocellular carcinoma (HCC), the risk of malignant transformation (estimated around 5 %), the risk of rupture (estimated around 30 %) with potential fatal consequences, and the fact that surgical excision guarantees a definitive diagnosis and long-term cure [42]. In a study with 74 resections for benign tumors, Terkivatan et al. [30] have recommended a resection for large (>5 cm) HA to reduce risk of rupture and malignant transformations. Deneve et al. [43] in a multicenter pooled analysis of 119 patients with HA undergoing surgical treatment during a 10-year period showed that the laparoscopic approach was adopted in 3 % of ruptured adenomas and in 11 % of not ruptured hematomas. Cho et al. [44] reviewed a large single-center series of 41 patients who underwent surgical resection for HA. The laparoscopic approach was limited to 9 patients (22 %) who were admitted in an elective setting with non-ruptured HA. In our experience of 15 pure LLR for HA [42], the indications for surgery were symptoms (n = 12), indeterminate character on imaging (n = 2), and size >5 cm (n = 1). The median tumor diameter was 85 mm (range 25–180), median length of stay was 4 days, and there was no mortality. Pure laparoscopic approach represents a safe and effective way for the surgical management of HA. Surgical resection ensures definitive diagnosis and treatment of HA with minimal risk of complications. This is particularly appealing for young individuals and for women who may worry about the risks of HA growth or rupture while using oral contraception or in future pregnancies.
19.2.3.4 Hepatic Cysts
Simple hepatic cysts are congenital hepatic lesions that are thought to result from progressive dilatation of biliary hamartomas, and they have no biliary communication. Their prevalence is estimated between 1.6 and 18 % [45]. Intracystic hemorrhage, secondary infection, and compression of the biliary tree are rare complications which may warrant a surgical intervention. If diagnosed with certainty, liver cysts must be managed conservatively regardless of their size. No follow-up is recommended because even if the cyst size increases, surgery will not be recommended if the patient remains asymptomatic.
19.2.3.5 Hepatobiliary Cystadenoma
Hepatobiliary cystadenomas are uncommon, benign cystic lesions of the biliary system and comprise less than 5 % of all hepatobiliary cystic masses. They can be serous or, more commonly, mucinous [46, 47]. Surgical resection is routinely indicated for fear of malignant transformation into a cystadenocarcinoma [48].
19.3 Preoperative Assessment
The evaluation of the patient undergoing LLR must include assessment of overall physical and functional fitness. LLR is an elective undertaking, and adequate optimization of patient’s medical condition should be achieved. Planning an LLR needs to take into account the nature of the lesion, its location, expected margins, liver anatomy and function, and volume of the future liver remnant (FLR). Good quality imaging is important prior to operative planning as variations in arterial, portal venous, and biliary anatomy are a frequent occurrence. Various clinical scoring systems (Child-Pugh’s score, Model for End-Stage Liver Disease score, etc.), dynamic liver tests (indocyanine green clearance, galactose elimination capacity, etc.), and volumetric assessments using imaging are helpful in preoperative planning. In high-risk surgical candidates, we routinely perform two dimensional echocardiography, pulmonary function test, and cardiopulmonary exercise test. Some patients (e.g., cirrhosis, nonalcoholic steatohepatitis [NASH], chemotherapy-associated steatohepatitis [CASH]) may have adequate volume but inadequate function and may experience liver failure if the FLR is too small [49]. In retrospective reviews evaluating outcomes of patients undergoing liver resection, the risk of death increases with decreasing volumes of the FLR [50, 51]. Therefore, assessment of operative risk prior to LLR includes establishing the severity of any possible ongoing liver disease. Patients with severe underlying functional liver disease or predicted low FLR are not surgical candidates. For patients who are candidates for LLR but who are deemed to have an inadequate FLR, strategies to induce FLR hypertrophy are to be considered (e.g., portal vein embolization). A frank conversation about the risk and benefits of LLR should be made with patients prior to any surgical plans.
19.4 Laparoscopic Liver Resections: Surgical Principles
19.4.1 Equipment and Manpower
Advanced laparoscopic surgery needs high-quality equipment and familiar operating room personnel to operate it. It is advisable that a trained scrub nurse and circulating nurse assistant dedicated to hepatobiliary surgery is available. A surgeon and an operating team should always ensure that a backup is handy in the event of equipment failure. A conventional instrument set for open hepatectomy should be handy as sometimes a conversion needs to be prompt. We prefer a 30° 10 mm camera and a pneumoperitoneum pressures up to 12–15 mmHg. We have had no problems of gas embolism with this strategy. We routinely use CUSA (Cavitron Ultrasonic Surgical Aspirator, Valleylab), intraoperative ultrasound, LOTUS (Laparoscopic Operation by Torsional Ultrasound, SRA Developments, Ashburton, Devon, UK), vascular clips, bulldog clamps, nylon slings, colored (red for arteries, blue for veins, and yellow for biliary structures) loops, disposable suction device, and disposable impermeable retrieval bags during LLR.
19.4.2 Preparation and Positioning
The patient is admitted on the day prior to surgery and reviewed by anesthetic and surgical teams. We maintain a dedicated LLR care pathway, and all the patients undergo standardized preparation. We do not administer intravenous hydration during the preoperative fasting. On the day of surgery, following endotracheal intubation and insertion of invasive monitoring lines (arterial line and central line), the surgical team inserts a urinary catheter and does abdominal wall shaving. It is important to shave the suprapubic area as that is the specimen retrieval site. All the patients are positioned in supine position on an adjustable table which can achieve longitudinal or sideway tilts. Inflatable calf compressors for deep vein thrombosis prophylaxis and Bair’s hugging forced-air warming blankets are routinely used. It is our preference to provide access to one of the arms, typically the left, to anesthetic team. Figure 19.4 explains our setup for a typical LLR case. It is important to ensure that all the ground wires are safely tapped to the floor to avoid accidental falls during movements.
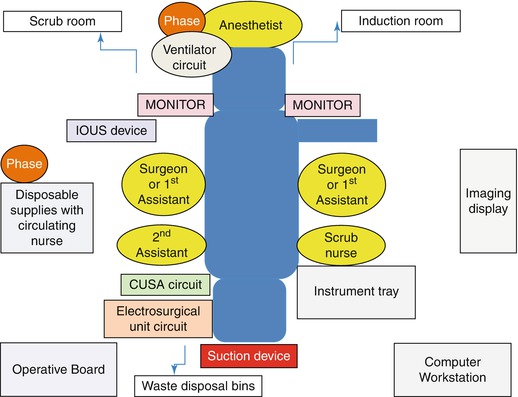

Fig. 19.4
Operating room setup for Laparoscopic Liver Resection at Southampton General Hospital
19.4.3 Anesthetic Conduct
Mechanical ventilation, pneumoperitoneum, and surgical manipulation of the liver reduce the hepatic blood flow. Hypovolemia and hypotension could result from head-up tilt and restrictive fluid strategy. The resultant reduction in cardiac output also reduces the hepatic blood flow. The anesthetist should focus on optimizing the oxygen delivery/oxygen extraction ratio by mitigating the above effects. Low central venous pressure anesthesia (0–5 mmHg) reduces bleeding from the hepatic veins. In instances of hemodynamic instability, a 15° Trendelenburg position could provide a rapid physiological bolus to maintain cardiac output transiently. This position improves venous return, preserves renal function, and reduces risk of gas embolism [52, 53]. Fluid replacement (“filling up” or “catching up”) is done after the resection is completed and hemostasis is achieved. Transesophageal echocardiography is sensitive to cardiac filling pressures and a useful adjunct.
19.4.4 Operative Technique
19.4.4.1 An Ideal Lesion
An ideal lesion for an LLR is solitary, small (<5 cm), and symptomatic and is located in the peripheral segments (II–VI) of the healthy liver. Pedunculated large lesions or lesions located in the segments II or III are also a good indication for LLR. However, one should question the indication of surgery when encountering such a patient with a benign disease, and surgery should only be considered when appropriate and after multidisciplinary team evaluation. When a hepatectomy is indicated in benign lesions, parenchyma-preserving surgery should be performed. Multiple lesions are suited if they can be resected en bloc with anatomical resection. Recently even lesions in the posterior section are approached laparoscopically; however, those are challenging resections and should be performed only by surgeons with advanced experience in LLR.
19.4.4.2 Basic Steps
The seven basic steps of LLR include:
1.
Port placement and peritoneal exploration
The number and position of ports will vary according to the type of resection which is planned, but it is our opinion that a surgeon should not hesitate to place an extra port if doing so facilitates the conduct of surgery.
2.
Liver mobilization
Once the ports are placed, the liver is mobilized along the falciform, triangular, and coronary ligaments as needed using ultrasonic dissectors or diathermy. It is important to have an astute assistant in order to avoid “manipulation injuries” or rupture of the lesions.
3.
Deciding the transection plane
Once the liver is mobile, the lesion is identified by correlating the preoperative imaging with intraoperative judgment. Intraoperative ultrasound is a useful adjunct, and we perform it routinely. This helps us in deciding the transection plane which is typically marked by electrocautery on the surface of the liver parenchyma (tattooing).
4.




Vascular inflow control
This is only needed in case of major anatomical resections and not in minor resections or left lobectomies. Selective inflow control may be achieved extrahepatically or intrahepatically. Extrahepatic control helps in demarcation of zone of ischemia which dictates the plane of transection. A laparoscopic surgeon must be familiar with variations in the biliary and vascular anatomy, and we consider it always a safe practice to do test clamps prior to sacrificing any large structure. This is akin to the manual palpation which is performed during an open resection. It is important to note that the first short branches from the portal vein are supplied to the caudate lobe (and hence it is termed as segment I) and a delicate dissection, isolation, and clipping or preservation should be performed depending on the intent of surgery. Intrahepatic inflow control can be performed after a hepatotomy near the hilum in proximity to the gallbladder fossa. It is our practice to routinely counsel patients for a cholecystectomy during an LLR and perform cholecystectomy selectively where the gallbladder is grasped for mobilization and exposure. Intrahepatic inflow control approach prevents injury to contralateral inflow but can result in bleeding. When vascular inflow need not be compromised prior to transection, e.g., planning a segmental resection, we perform temporary clamping, i.e., Pringle’s maneuver. Typically this is secured via the nylon sling via the left flank 5 mm port and controlled extracorporeally by the assistant. This helps us in reducing the blood loss. We perform Pringle’s clamp in cycles of “10 min on” and “5 min off,” and this time is typically maintained by anesthetic colleague. Compared to continuous clamping, intermittent clamping is shown to reduce ischemia/reperfusion injury and reduction in elevation of liver enzymes postoperatively [54]. In an attempt to reduce the ischemic insult associated with Pringle’s maneuver, ischemic preconditioning is proposed [55]. It refers to the brief interruption of blood flow, followed by a short reperfusion interval, and then a more prolonged period of ischemia. Once the inflow is controlled, we aim for parenchymal transection.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree






