Chapter 34 Laparoscopic cholecystectomy and choledocholithotomy
Overview
Gallstones are an extremely common condition, occurring in approximately 10% to 20% of the adult population. It has long been recognized that the treatment for symptomatic gallstones is cholecystectomy, one of the most commonly performed abdominal surgical procedures. In developed countries, most cholecystectomies are performed laparoscopically. To give an example, 90% of cholecystectomies in the United States are performed laparoscopically (Csikesz et al, 2009). Laparoscopic cholecystectomy is considered the gold standard for the surgical treatment of gallstone disease, because it results in less postoperative pain, better cosmesis, shorter hospital stays, and less disability when compared with open cholecystectomy (Soper et al, 1992c). The early experience with laparoscopic cholecystectomy was associated with an increased risk for common bile duct (CBD) injuries. However, through advances in education and training, this risk has been decreased. In the hands of properly trained surgeons, laparoscopic cholecystectomy can be safely performed in the majority of patients.
Over the past several years, much investigation has been done on techniques to improve outcomes from laparoscopic cholecystectomy. One approach has been to use smaller diameter ports and instruments to perform the operation (Gurusamy et al, 2010); however, the limited variety of 2 mm diameter instruments and their lack of robustness have hindered this approach. More recently, there has been an upsurge in interest in the performance of laparoscopic cholecystectomy using a single incision at the umbilicus or via natural orifice translumenal endoscopic surgery (NOTES). Up to this time, no study has definitely proven the advantage of either smaller incisions or fewer incisions compared with standard multiport laparoscopic cholecystectomy, other than the potential for improved cosmesis. This chapter discusses the history and current status of laparoscopic cholecystectomy and the laparoscopic management of CBD stones.
History
Gallbladder disease has plagued humanity since antiquity, and gallstones have been found in Egyptian mummies dating from more than 2000 years ago (Beal, 1984). Some have even conjectured that the death of Alexander the Great was caused by acute cholecystitis. Although the presence of the gallbladder was acknowledged by anatomists beginning in the fifth century, the gallbladder’s true function was not elucidated until relatively recently. The potential for elective surgical treatment of gallstones was limited by the crude diagnostic techniques available, but the first successful cholecystolithotomy likely occurred in 1676, when Joenisius apparently extracted gallstones from a biliary fistula of the abdominal wall (Beal, 1984). By the eighteenth century, physicians increasingly realized that cholelithiasis could cause abdominal pain and jaundice. As surgical treatment was not considered to be feasible, numerous treatments were described, including purgatives, therapeutic bleeding, and emetics. It was not until 1867 that John S. Bobbs of Indianapolis performed an elective cholecystotomy for hydrops of the gallbladder under chloroform anesthesia (Cutter, 1928). Various other surgeons then described techniques for performing cholecystolithotomy in this era, as Listerian principles were being adopted around the world.
Carl Langenbuch of Berlin performed the first elective cholecystectomy in 1882 on a patient who had been suffering from symptomatic cholelithiasis for more than 10 years. Although the patient was found “smoking a cigar” the following day, he was not discharged from the hospital for two months. Dr. Langenbuch stated, “My opinion is that a cholecystectomy is suitable for those cases in which both the patient and physician have reached the end of their patience” (Langenbuch, 1882). The first cholecystectomy was not performed in the United States until 1886, by Justus Ohage of Minnesota. For the next 30 years, considerable debate followed at surgical meetings as to whether a cholecystolithotomy or cholecystectomy would be preferable for gallstone disease. By the 1920s it was generally accepted that cholecystectomy was most suitable, except in the face of severe inflammation, rendering the dissection hazardous to the patient.
Over the ensuing 6 decades, open cholecystectomy was the procedure of choice for patients with symptomatic cholelithiasis. Great strides were made in developing diagnostic methods for demonstrating the presence of gallstones, using either cholecystography or ultrasound (US). Throughout much of this time, many of the operations were done by family physicians not well trained in surgical techniques, and thus bile duct injuries were seen relatively frequently. In Christopher’s Textbook of Surgery from 1936 it states, “Frank hemorrhage from the cystic artery and injury to the CBD are the two great dangers in performing cholecystectomy. Cholecystectomy will not only be easier but safer if it is begun at the cystic duct, since the circulation will be controlled at the outset.” Later, other surgeons suggested that a top-down or fundus-first technique for performing cholecystectomy would be less likely to result in CBD injury. Various surgical debates included whether to perform intraoperative cholangiography on a routine or selective basis and the appropriate timing for performing cholecystectomy in the face of acute cholecystitis. Some surgeons advocated performing cholecystectomy via a small incision (minicholecystectomy) to minimize the discomfort and disability brought about by larger incisions during gallbladder removal (Basu et al, 2006).
By the 1980s, the rates of morbidity and mortality for open cholecystectomy were quite acceptable. In published reports of large series taken from population-based studies or from single institutions, mortality rates ranged from 0.1% to 0.6%, and overall morbidity was 10% to 15% with bile duct injuries occurring in 0.1% to 0.2% of patients. It was also shown clearly that complications occurring with open cholecystectomy were directly related to the condition of both the patient and the gallbladder at the time of operation. Postoperative hospital length of stay for open cholecystectomy varies widely, but most elective cases are discharged within 4 days of operation (Hall, 1987).
Medical Therapy for Gallstone Disease
The prevailing public perception of open cholecystectomy as a procedure that results in pain, disability, and a disfiguring scar engendered many attempts in the 1980s and 1990s at nonoperative treatment of gallstones (Schoenfield & Lachin, 1981; Schoenfield et al, 1990). In addition, an understanding of the chemistry of gallstones led to numerous attempts to develop agents that could dissolve cholesterol gallstones. By the mid-1980s, ursodeoxycholic acid was made commercially available for oral administration, and its use was described in several clinical studies. Unfortunately, the indications for this agent were limited, and the 40% rate of complete gallstone dissolution in this highly selected group was disappointing (Roda et al, 1982). In the late 1980s gastroenterologists and interventional radiologists tried to increase this dissolution rate by attempting direct contact dissolution of gallstones. The biliary tree was accessed percutaneously, and methyl tert-butyl ether (MTBE) was instilled in the gallbladder. This therapy was invasive and had significant side effects, thus it was rapidly abandoned (Thistle et al, 1989).
In the early 1980s, extracorporeal shock wave lithotripsy (ESWL) was shown to be efficacious therapy for kidney stones. Others extrapolated that shock wave therapy might also break gallstones into smaller fragments that could then be more effectively dissolved. A brief flurry of interest and activity in the use of ESWL for gallstones followed, but the selection criteria limited this to fewer than 20% of gallstone patients. Despite strict selection criteria, the efficacy of treatment at 6 months was only 22%, and the gallstone recurrence rate of 10% per year for the first 5 years left much to be desired (Sackmann et al, 1990).
Laparoscopic Cholecystectomy
The technique of laparoscopy (lapara, the flank; skopein, to examine) was initially developed near the outset of the twentieth century. However, until the 1960s, its use was strictly limited to diagnostic procedures within the peritoneal cavity. Gynecologists first used laparoscopic techniques to perform tubal ligation. By that time, the laparoscopic optics, light sources, and carbon dioxide insufflators had been markedly improved, but the eyes of the surgeon were wedded to the eyepiece of the laparoscope during performance of the operation. Despite this limitation, Dr. Kurt Semm of Kiehl, Germany, the father of “pelviscopy,” performed the first laparoscopic appendectomy in 1980 (Semm, 1982). By 1983, Semm stated that “laparoscopic cholecystectomy and the bowel anastomosis under laparoscopic vision had moved into the domain of the possible” (Manegold, 1985). Two years later, a German surgeon named Eric Muhe working in Böblingen, West Germany, performed the first laparoscopic cholecystectomy (Litynski, 1996). He used a modified operating laparoscope placed at the umbilicus after establishing pneumoperitoneum. Muhe later changed his technique to perform cholecystectomies through a small, open tube placed in the right upper quadrant without pneumoperitoneum. Despite presenting his initial work at the congress of the German Surgical Society in 1986, Muhe’s approach was rejected by German surgeons and largely forgotten. In 1987 a French surgeon, Phillipe Mouret of Lyon, France, performed laparoscopy on a woman with both gallstones and a gynecologic disorder. He performed the first video laparoscopic cholecystectomy by using a camera attached to the laparoscope, thereby allowing the entire operating team to view the operative field. As he was in private practice, few other surgeons learned of his work initially, but word spread within France. The following year both François DuBois in Paris and Jacques Perissat in Bordeaux, working in academic centers, performed laparoscopic cholecystectomy. Relatively soon thereafter, two groups of general surgeons in the southern United States performed laparoscopic cholecystectomy. They used a laser to dissect the gallbladder away from the liver bed, as it was thought that monopolar electrocautery used within the closed abdomen was potentially a hazard for combustion. The first publication of the “laparoscopic laser cholecystectomy” was published in the industry publication “Laser Practice Report” by Reddick in December 1988, whereas DuBois and colleagues published their first 36 cases (in English) in 1990.
All of these early pioneers in laparoscopic cholecystectomy used a four-port technique with the laparoscope placed at the umbilicus, albeit the French surgeons stood between the patient’s legs, and the Americans worked from the patient’s left side. Word of this new laparoscopic operation spread relatively rapidly after its introduction at the Society of American Gastrointestinal and Endoscopic Surgeons (SAGES) meeting in Louisville in April 1989, and the subsequent American College of Surgeons meeting in Atlanta in October 1989. Given that cholecystectomy makes up nearly a third of the work in many general surgeons’ practices, surgeons in the United States feverishly attempted to learn this technique for a competitive advantage. Numerous courses sprang up across the United States and Europe. Many of these courses were sponsored by industry and provided little hands-on experience for surgeons who generally were poorly trained in basic laparoscopic techniques. It must be remembered that laparoscopy differs significantly from traditional open surgery in several important ways: 1) a satisfactory pneumoperitoneum of carbon dioxide gas must be established in the abdominal cavity; 2) the laparoscopic image of the operative field is controlled by someone other than the surgeon, and this image is highly magnified and two-dimensional; 3) the surgeon views a video screen, rather than the operative field itself; and 4) the laparoscopic ports act as a fulcrum, so the tips of the instruments move in the direction opposite the surgeon’s hands. Given these and other differences, it is not surprising that a learning curve effect was quickly recognized, whereby the incidence of bile duct injuries was much higher in a surgeon’s early cases compared with later in his or her experience (Meyers & Southern Surgeons Club, 1991).
Going from an unheard of procedure in 1988 to one that had become the subject of numerous published case series by 1992, the adoption rate of this technology in the United States was unprecedented. In 1992, the National Institutes of Health (NIH) organized a consensus development conference titled “Gallstones and Laparoscopic Cholecystectomy,” which was sponsored by the National Institute of Diabetes and Digestive and Kidney Diseases and the NIH office of Medical Applications of Research. At the conclusion of the conference, it was determined that laparoscopic cholecystectomy “provides a safe and effective treatment for patients with symptomatic cholelithiasis” and “provides distinct advantages over open cholecystectomy” but that “the outcome of laparoscopic cholecystectomy is influenced greatly by the training, experience, skill, and judgment of the surgeon performing the procedure” (Gollen et al, 1993).
It was also in 1992 that the first of several small prospective randomized trials comparing laparoscopic to open cholecystectomy was published. Each of these showed that the laparoscopic approach was associated with less pain, shorter hospitalization, and a more rapid return to full activity (Barkun et al, 1992). Coincidentally, 1992 was also the year in which a publication of a large series of laparoscopic cholecystectomies first suggested that this technique may become the new gold standard for performance of the operation (Soper et al, 1992b). By contrast, a relatively large randomized trial comparing laparoscopic with open cholecystectomy using a minilaparotomy showed no difference in many standard outcome measures (Majeed et al, 1996).
Over the ensuing years, a number of trials confirmed what had previously been shown in the open cholecystectomy literature. The aphorism “get it while it’s hot” could be used to describe the preferred approach to laparoscopic cholecystectomy for acute cholecystitis: prospective randomized trials showed that early cholecystectomy resulted in fewer complications and shorter hospitalization than interval cholecystectomy (Lo et al, 1998). Intraoperative cholangiography was performed on a selective basis by the majority of practicing surgeons, despite several population-based studies showing decreased rates of bile duct injury when performed by surgeons using cholangiography routinely during laparoscopic cholecystectomy (Flumm & Massarweh, 2007). Numerous reports were published concerning the causes of bile duct injury during laparoscopic cholecystectomy and the means by which these injuries could be minimized, including dissecting “the critical view of safety” before clipping or dividing the cystic structures (Strasberg et al, 1995).
Indications
An increase in the number of cholecystectomies performed has been documented since the introduction of laparoscopic cholecystectomy (Escarce et al, 1995; Legorreta et al, 1993; Nenner et al, 1994; Steiner et al, 1994). It is unclear whether patients are more willing to undergo a laparoscopic procedure, rather than to endure biliary colic, or whether the indications for cholecystectomy have become more liberal with the advent of laparoscopic cholecystectomy.
The indications for laparoscopic cholecystectomy are, and should be, the same as the indications for open cholecystectomy (Table 34.1). Patients generally have documented cholelithiasis and symptoms attributable to a diseased gallbladder (see Chapter 30). Biliary colic is typically a severe and episodic right upper abdominal or epigastric pain that often radiates to the back. Attacks frequently occur postprandially, or they awaken the patient from sleep. Patients with asymptomatic gallstones have less than a 20% chance of ever developing symptoms, and the risks associated with prophylactic operation outweigh the potential benefit of surgery in most patients (Fendrick et al, 1993; Ransohoff & Gracie, 1993; Ransohoff et al, 1983). Prophylactic laparoscopic cholecystectomy for asymptomatic cholelithiasis may be justified for certain patients, however, such as those with sickle cell disease, those who require long-term total parenteral nutrition, and those who are therapeutically immunosuppressed after solid organ transplantation. Patients with sickle cell disease often have hepatic or vasoocclusive crises, which can be difficult to differentiate from acute cholecystitis (Tagge et al, 1994). In transplant patients, there is concern that immunosuppression would mask the signs and symptoms of inflammation until overwhelming infection had occurred (Hull et al, 1994). Recommendations in the literature range from mandatory screening and treatment of biliary disease before transplantation (Girardet et al, 1989) to prophylactic cholecystectomy 6 months after transplantation (Boline et al, 1991) to expectant management of all asymptomatic patients (Fendrick et al, 1993; Steck et al, 1991). Other possible indications for prophylactic laparoscopic cholecystectomy include known gallstones in patients who may not have access to modern health care facilities for an extended period, such as missionaries and military personnel, and patients who already are undergoing laparoscopic abdominal surgery for other indications (incidental laparoscopic cholecystectomy). Less commonly, individuals without gallstones but with typical biliary symptoms (i.e., acalculous cholecystitis or biliary dyskinesia) may be considered for the procedure (Soper, 1991). Other indications for laparoscopic cholecystectomy include gallstone pancreatitis and gallbladder polyps greater than 1 cm in size (see Chapter 49).
Table 34.1 Indications for Laparoscopic Cholecystectomy
Contraindications
Absolute and relative contraindications to performing laparoscopic cholecystectomy (Table 34.2) have decreased since the 1990s, as minimally invasive surgical equipment and skills have improved. Absolute contraindications include the inability to tolerate general anesthesia or laparotomy, refractory coagulopathy, diffuse peritonitis with hemodynamic compromise, cholangitis, and potentially curable gallbladder cancer. Diffuse peritonitis with hemodyInamic compromise represents a surgical emergency in which attempted laparoscopic cholecystectomy is not prudent, because the cause of the peritonitis is unclear or uncertain. Standard open laparotomy allows rapid determination of the cause and more expeditious management of the disorder, and pneumoperitoneum may exacerbate hypotension by diminishing venous return. Suspicion of gallbladder malignancy generally mandates that standard open resection be undertaken; this is because of persistent concerns with adequacy of resection and reports of port-site metastases associated with the use of minimally invasive surgical techniques for the treatment of intraabdominal malignancies (see Chapter 49). Relative contraindications are dictated primarily by the surgeon’s philosophy and experience. These include previous upper abdominal surgery with extensive adhesions, cirrhosis, portal hypertension, severe cardiopulmonary disease, morbid obesity, and pregnancy.
Table 34.2 Contraindications to Laparoscopic Cholecystectomy
| Absolute |
| Relative |
Previous Abdominal Surgery
Intraabdominal adhesions secondary to previous abdominal surgery can tether underlying viscera and consequently increase the risk of hollow organ injury during placement of laparoscopic trocars (Wolfe et al, 1991). Previous lower abdominal operations typically add little difficulty in performing cholecystectomy, but patients with previous upper abdominal operations, especially in the right upper quadrant, present much more difficulty in gaining access to the area around the gallbladder. To diminish the risk of intraperitoneal injury, we routinely use the open Hasson technique for placement of the initial trocar. Other surgeons advocate the use of the Veress needle and percutaneous insertion of the initial trocar at a site remote from the abdominal wall scars. Once the initial trocar is placed, additional trocars are placed under direct laparoscopic visualization in an area free of adhesions. Some degree of laparoscopic adhesiolysis may be necessary to allow optimal port placement. Although early series demonstrated that previous abdominal surgery with the accompanying risk of adhesions was a predictor of conversion, it is clear that laparoscopic cholecystectomy may be performed safely on most of these patients.
Cirrhosis
Cirrhosis (see Chapter 70B) results in a brittle, friable, heavy liver that may be difficult to retract in the cephalad direction, limiting exposure of the porta hepatis and gallbladder. Cirrhosis also may be accompanied by decreased synthetic function of the liver, resulting in coagulopathy and portal hypertension. Coagulopathies should be reversed before performance of laparoscopic cholecystectomy.
The ability to achieve effective hemostasis laparoscopically is significantly compromised compared with that of open exposure. Portal hypertension and aberrant portosystemic venous collateralization may lead to exsanguinating hemorrhage from small veins in the liver bed and porta hepatis or from large veins in the abdominal wall (e.g., a recanalized umbilical vein) at risk for laceration during trocar puncture. Laparoscopic cholecystectomy may be attempted with care in these patients by experienced surgeons. Various hemostatic agents and energy sources need to be available in case bleeding develops; the argon cautery device can be quite useful. Prompt conversion to an open procedure is recommended in the face of unusual bleeding, regardless of the stage of the operation (Soper, 1993). Additionally, particular care should be taken in making watertight closures for all incisions to minimize postoperative ascitic leak (Poggio et al, 2000).
Cardiopulmonary Disease
Most patients undergoing laparoscopic cholecystectomy exhibit mildly elevated Pco2 (Liu et al, 1991). Chronic obstructive pulmonary disease may predispose patients to carbon dioxide retention disproportionate to the measured end-tidal value during laparoscopic cholecystectomy (Wittgen et al, 1991). Prudent measures in these patients include obtaining preoperative pulmonary function tests, making arterial blood gas determinations, and maximizing pulmonary function by smoking cessation and bronchodilator therapy. An intraoperative arterial catheter also should be placed to allow frequent measurement of Pco2 and pH. Hypercarbia may manifest as hypertension, tachycardia, or ventricular arrhythmias; these should be addressed by immediate evacuation of the pneumoperitoneum and stabilization. Gradual reestablishment of the pneumoperitoneum with a low pressure limit may be attempted, but the procedure should be terminated or converted to open if the hypercarbia recurs and is refractory to continued pneumoperitoneum.
Morbid Obesity
Other challenging factors associated with morbid obesity include the presence of an enlarged, friable, fatty liver and an increased amount of adipose tissue around the gallbladder and in the area of the triangle of Calot. The surgeon should not hesitate to place additional trocars if needed (Schirmer et al, 1992). Retrospective and prospective studies have shown modestly increased operative times with performance of minimally invasive procedures in obese patient groups (Schirmer et al, 1992; Underwood et al, 1998). Today, laparoscopic cholecystectomy is commonly performed in morbidly obese patients and seems to offer the same advantages as in nonobese patients; it may also offer advantages more specific to obese patients, such as a decrease in wound infections, incisional hernias, and thrombotic complications (Miles et al, 1992; Talamini & Gadacz, 1992). Rather than being contraindicated in morbidly obese patients, laparoscopic cholecystectomy may become the preferred mode of therapy for these patients.
Pregnancy
Studies have shown that laparoscopic cholecystectomy can be performed safely during pregnancy, but only with great care (Soper et al, 1992a); A retrospective cross-sectional analysis of hospital discharge data from the Healthcare Cost and Utilization Project Nationwide Inpatient Sample database was conducted to study outcomes after cholecystectomy during pregnancy (Kuy et al, 2009). In this study, 9714 pregnant women who underwent cholecystectomy experienced significantly higher surgical, maternal, and fetal complication rates, lengths of stay, and costs with open cholecystectomy compared with laparoscopic surgery (Kuy et al, 2009). This may reflect the reasons for choosing an open cholecystectomy—such as prior surgery, gangrenous gallbladder, or trimester of pregnancy—rather than a true difference in complication rates between the procedures. High-volume surgeons, defined as those providers above the 75th percentile based on the annual number of cholecystectomies performed, had significantly lower rates of surgical complications.
Preoperative Evaluation and Preparation
Laboratory Test and Imaging Studies
Patients undergoing elective laparoscopic cholecystectomy for biliary colic should have preoperative liver function tests (LFTs). Elevations in these tests suggest the presence or recent passage of CBD stones. In patients with cholecystitis, LFTs are not typically elevated, because the obstruction is limited to the gallbladder; if present, elevated LFTs should raise concerns about complicating conditions such as cholangitis, choledocholithiasis, or Mirizzi syndrome (a gallstone impacted in the distal cystic duct causing extrinsic compression of the CBD; see Chapter 35).
In a patient with typical biliary colic, the only diagnostic study necessary before laparoscopic cholecystectomy is an abdominal US revealing gallstones. US shows the size and number of stones, the thickness of the gallbladder wall, the presence or absence of pericholecystic fluid, and the diameter of the CBD and other components of the biliary ductal system (see Chapter 13). Other nonbiliary disorders, such as hepatic lesions or steatosis, masses in the pancreas, or renal tumors, also may be diagnosed. When US results are negative despite typical biliary symptoms, cholecystokinin-stimulated biliary scintigraphy showing a low gallbladder ejection fraction with or without pain reproduction suggests acalculous cholecystitis or gallbladder dyskinesia (Soper, 1993).
If a patient with gallstones has atypical symptoms, however, a more extensive workup that includes upper gastrointestinal contrast radiography or endoscopy, computed tomography (CT), or cardiac and pulmonary evaluation may be appropriate to rule out significant nonbiliary disease processes. Magnetic resonance cholangiopancreatography (MRCP) may be useful to evaluate the common duct in patients with mild elevations of their transaminases or mild CBD dilatation on US (see Chapter 17). Additionally, if a patient has a dilated CBD, CBD stones, or jaundice, consideration should be given to a preoperative endoscopic retrograde cholangiopancreatography (ERCP) with clearing of the stones followed by laparoscopic cholecystectomy (see Chapter 18).
Antibiotics
Routine preoperative antibiotic administration for elective laparoscopic cholecystectomy is controversial, with strong opinions on both sides. A recent meta-analysis of randomized controlled trials concluded that prophylactic antibiotics do not prevent infections in low-risk patients undergoing laparoscopic cholecystectomy; the usefulness of prophylaxis in high-risk patients—those older than 60 years and those with diabetes, acute colic within 30 days of operation, jaundice, acute cholecystitis, or cholangitis—remains uncertain (Choudhary et al, 2008). The most recent randomized, prospective study included in the above-mentioned meta-analysis showed no difference in the postoperative wound infection rate, although the control group had a 1.5% infection rate, and the antibiotic group had a 0.7% infection rate; however, because there was a total of 277 patients in the study, a type II error might have been made (Chang et al, 2006). Among papers that suggest a benefit of antibiotic prophylaxis is a recent randomized study that found fewer wound infections with ampicillin-sulbactam versus cefuroxime, particularly for infection caused by Enterococcus in the setting of high-risk patients undergoing elective cholecystectomy (Dervisoglou et al, 2006).
Operative Technique
Operating Room Setup
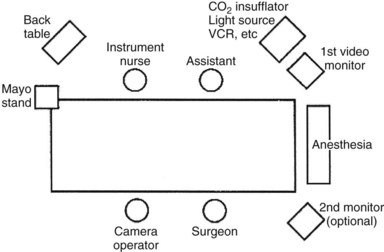
Using the “American” technique, the surgeon stands to the left of the patient, the first assistant stands to the patient’s right, and the laparoscopic video camera operator stands to the left of the surgeon (Fig. 34.1). In the “French” technique, the patient’s legs are abducted, and the surgeon stands between them. The camera operator always must maintain the proper orientation of the camera and keep the operating instruments in the center of the video image. Following all instruments as they enter or exit the operative field is a matter of surgeon preference. Sharp instruments should never be moved intracorporeally unless they are under direct videoscopic vision.
Pneumoperitoneum
A working space, generally provided by a pneumoperitoneum, is essential for the surgeon to both view and operate within the abdominal cavity. Carbon dioxide has the advantage of being noncombustible and rapidly absorbed from the peritoneal cavity; however, it may lead to hypercarbia in patients with significant cardiopulmonary disease (Fitzgerald et al, 1992). Pneumoperitoneum can be established by either a closed or an open technique. In the closed technique, carbon dioxide is insufflated into the peritoneal cavity through a Veress needle placed blindly into the abdominal cavity; the needle is subsequently replaced with a laparoscopic port. In the open technique, a laparoscopic port is inserted under direct vision into the peritoneal cavity via a small incision; pneumoperitoneum is established only after ensuring definitive and safe peritoneal entry. Both techniques have advantages and disadvantages, and surgeons performing laparoscopic cholecystectomy should learn both techniques and use them selectively.
Insufflator tubing is connected to the Veress needle, and carbon dioxide is insufflated at a low flow rate of approximately 1 L/min. The initial pressure of the abdomen is usually 2 to 6 mm Hg and should not increase appreciably during the early phase of insufflation. Asymmetric distension, a rapid increase in pressure with low insufflated volume, or an initial pressure greater than 10 mm Hg suggests that the needle is not in the proper position. The abdomen should be serially percussed to confirm symmetric tympany associated with insufflation. The abdomen is fully insufflated with the upper pressure limit set at 12 to 15 mm Hg; this usually requires 3 to 6 L of carbon dioxide, depending on the size of the abdominal cavity and degree of muscle relaxation. If intraabdominal pressures exceed 20 mm Hg, central venous pressures and blood pressures decrease because of decreased venous return and diminished cardiac output (Chui et al, 1993; Sharma et al, 1997). Studies also have shown direct negative effects on urine output relative to increased intraabdominal pressures (McDougall et al, 1996, 1997).
During the initial period of insufflation, the patient must be monitored closely for signs of gas embolism (hypotension, decreased oxygen saturation, decreased end-tidal carbon dioxide, “mill-wheel” heart murmur), vagal reaction (hypotension, bradycardia), ventricular arrhythmias, and hypercarbia with acidosis. Most of these complications require immediate treatment by desufflation followed by gradual reestablishment of the pneumoperitoneum after the patient’s condition has stabilized. Gas embolism results in an “air lock” right ventricular outflow obstruction with a dramatic decrease in end-tidal carbon dioxide concentration. When suspected, this problem should be treated by placing the patient in a steep Trendelenburg and left lateral decubitus position followed by insertion of a central venous catheter to aspirate the carbon dioxide from the apex of the right ventricle (Chui et al, 1993; Hanney et al, 1995; Lantz & Smith, 1994; Sharma et al, 1997).
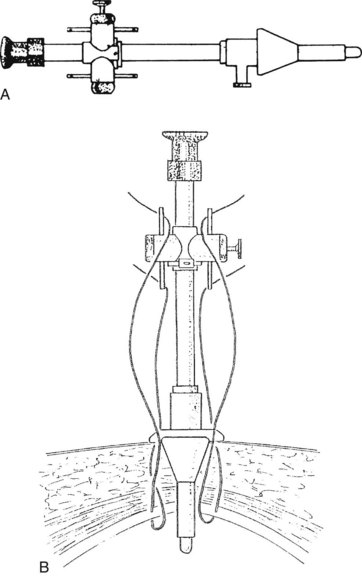
Using the open technique, the pneumoperitoneum is established similarly to an open diagnostic peritoneal lavage. A 1.5-cm skin incision is made in the infraumbilical or supraumbilical skin fold. Dissection of the subcutaneous tissue is performed at the base of the umbilical raphe to reach the fascia rapidly, even in obese patients, because this is the thinnest part of the abdominal wall. Kocher clamps are applied to both sides of the linea alba, and a small vertical incision is made to gain access to the peritoneal cavity. A finger is placed into the wound to ensure that the free peritoneal cavity has been entered and to sweep away any adhesions that may be present. If a Hasson trocar (Weck & Co, Research Triangle Park, NC) is available, two sutures are placed on both sides of the fascial incision and are tied to the wings of the wedge-tipped sheath after it is inserted into the peritoneal cavity under direct vision (Fig. 34.2). Alternatively, standard laparoscopic sheaths can be used after placing two concentric polypropylene purse-string stitches around the fascial incision. After removing the sheath at the conclusion of the procedure, the outer purse-string suture is removed, and the inner one is tied. Open insertion of the initial port takes a few minutes longer than its closed counterpart. Extraction of the gallbladder at the conclusion of the operation is easier, however, which equalizes the time differential of the two access techniques.