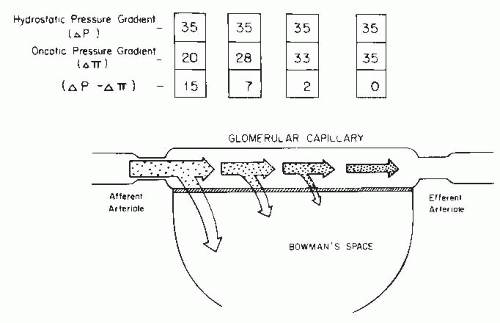
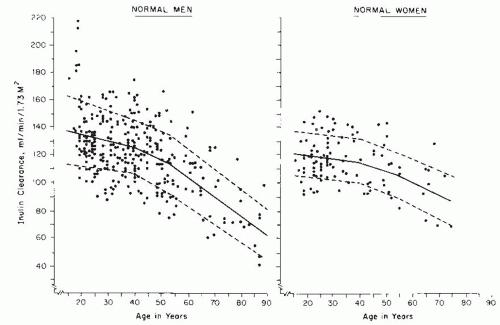
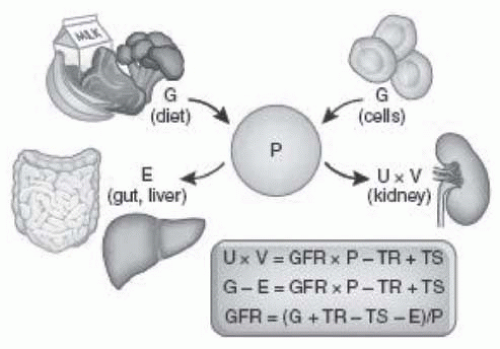
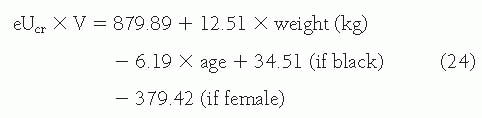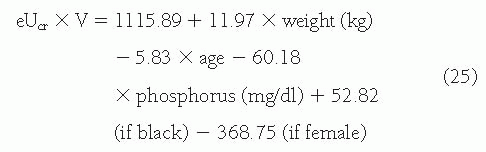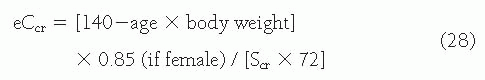Normal Range and Variability of Glomerular Filtration Rate
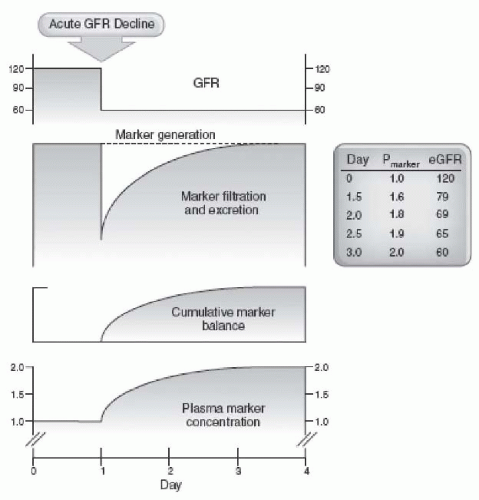
The GFR cannot be measured directly. Instead, as discussed later, it is assessed from the urinary clearance of an ideal filtration marker, such as inulin. When measured repeatedly in a single individual, under constant conditions and according to a standard protocol, the GFR appears relatively constant. Homer Smith measured the inulin clearance in one “hospitalized but otherwise normal subject” 15 times during 1 year; the range was 113 to 137 mL per minute with a mean of 122 mL per minute.
15 However, variation among individuals is quite large, and normal values show considerable spread. As discussed later, the major causes of variability in healthy individuals are age, gender, and body size. Hence,
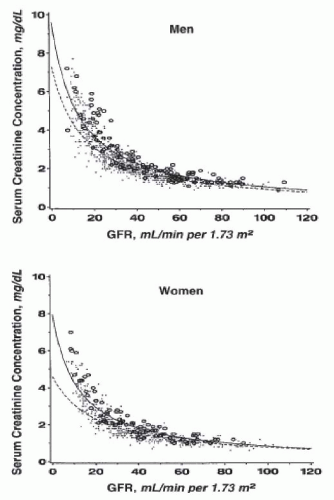
measured values of GFR are typically adjusted for body size (surface area) and are traditionally compared to normative values for age and gender (
Fig. 9.5).
16 Even after elimination of these sources of variation, important variability remains. A compilation of inulin clearance measurements in hydrated young adults (adjusted to a standard body surface area of 1.73 m
2) shows the mean value in men to be 131 mL per minute with a coefficient of variation (CV) (defined as the standard deviation divided by the mean) of 18%, and the mean value in women to be 120 mL per minute, with a CV of 14%.
15,
16 The following sections discuss causes of normal variation. These same factors also contribute to variation in GFR in patients with kidney disease.
Age, Sex, Body Size, and Ethnicity. The surface area adjustment was first introduced to minimize variability in urea clearance results among normal adults and children.
17,
18,
19 Based on the relationship of GFR to glomerular surface area, it is not surprising that the level of GFR is related to kidney size, which in turn, is related to body surface area and metabolic activity.
20 Measured values for GFR are conventionally factored by 1.73 m
2, the mean surface area of men and women 25 years of age. Nonetheless, as described earlier, surface-area adjusted values for GFR are approximately 8% higher in young men than in women of the same age.
Glomerular tuft volume, renal size, and GFR increase during growth and development. The surface area adjustment is not appropriate for newborns, whose adjusted GFR is less than 50% of the value achieved at approximately 1 year of age.
21,
22 More recent studies strongly suggest that in newborns, GFR should be expressed in mL/min/kg, with the normal value being 0.6 to 1.6 mL/min/kg. Such an approach reduces the apparent variation in measured GFR more than 10-fold.
23 Beyond age 1 to 2 years, however, GFR values in normal children, adjusted to 1.73 m
2, are the same as those for young adults.
The appropriateness of the surface area correction in obesity remains controversial.
24 Because adipose tissue is less metabolically active than lean body mass, the physiologic matching of GFR to body surface area may not be the same in obese as in lean individuals. There are few data to relate measured GFR to body size, metabolic activity, and risks for development of kidney disease in obesity, leaving many important questions unanswered.
25 Are estimates of body surface area from height and weight as accurate in obese as in lean individuals? Does GFR increase with weight gain in proportion to body surface area? If so, is the resulting hyperfiltration associated with increased risk for development of kidney disease, as hypothesized in other conditions with hyperfiltration, such as diabetes? If so, indexing GFR to body surface area in obesity may obscure detection of an important marker of disease. Cross-sectional and longitudinal studies of measured GFR in obesity, in association with measures of body size and metabolic activity, and markers of kidney damage are necessary to answer these questions.
24Most studies of measured GFR in populations without kidney disease have been conducted in North America or
Europe, so data on nonwhite races and other ethnicities is limited. Reports on small to moderate numbers of subjects have suggested a lower average value,
26,
27 but these studies are somewhat limited by differences in GFR measurement methods and by incomplete ascertainment of protein intake (see below). A more recent report from a representative population in Pakistan suggests mean values of GFR in young adults only slightly below those in whites, with similar agerelated decline.
28In older studies, both cross-sectional and longitudinal studies in normal men demonstrate an age-related decline in GFR of approximately 10 mL/min/1.73 m
2 per decade after the age of 30 years.
16,
29,
30,
31 Recent studies in the general population have not been performed, but studies in kidney donors demonstrated a 4 mL/min/1.73m
2 lower measured GFR per decade up to the age of 45 years and a 7.5 mL/min/1.73m
2 lower measured GFR per decade thereafter.
32 Thus, using the data from the general population, during the 50 years from age 30 to 80, GFR declines by almost 40% from approximately 130 to 80 mL/min/1.73 m
2. Crosssectional studies in normal women indicate roughly similar results, but comparable longitudinal studies have not been performed and there may be subtle differences related to effects of hormones, pregnancy, and propensity toward illnesses that impact the kidney. This age-related decline in GFR is consistent with the anatomic observation that the number of glomeruli in the normal human kidney declines with age; in the sixth and seventh decades, the number of glomeruli is less than one-half the number present in young adults.
4,
33 The cause of age-related decline in GFR is not completely understood, but progressive glomerular sclerosis, independent of traditional kidney disease risk factors, likely contributes to the loss of glomeruli.
34,
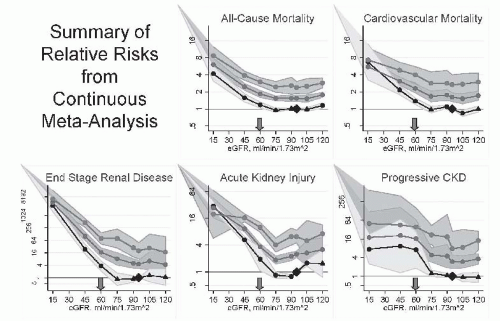
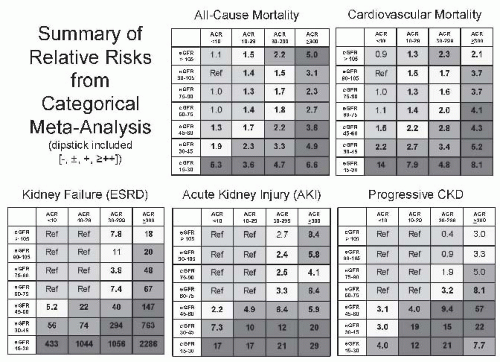
35 Recent epidemiologic studies demonstrate that this decline in GFR is associated with increased risk for all-cause and cardiovascular disease mortality as well as kidney disease, casting doubt on the traditional interpretation that it is normal.
36Pregnancy. Marked increases in GFR occur during pregnancy; elevations to an average as much as 50% occur during the first trimester, and these high levels persist until shortly after term.
37,
38,
39,
40 These increments in GFR are associated with an increase in renal plasma flow and relatively constant filtration fraction throughout most of pregnancy, reflecting hemodynamic consequences of widespread vasodilatation. Late in pregnancy, it appears that hyperfiltration becomes dependent on reduced plasma oncotic pressure. This change persists in the very early postpartum period, but the GFR returns to normal in the first 4 to 8 weeks following the end of pregnancy.
40,
41
Interestingly, pregnancy-induced hyperfiltration also occurs in women with preexisting chronic kidney disease.
42 This observation suggests that the physiologic vasodilatation of pregnancy can further augment the single-nephron hyperperfusion and hyperfiltration associated with chronic kidney disease. However, this phenomenon may be restricted to women with only very mild reductions in GFR. Improvement of GFR was not observed in one study of 23 women with chronic kidney disease and pre-pregnancy serum creatinine levels greater than 1.4 mg/dL.
43Protein Intake. The effect of protein intake to modulate GFR in experimental animals was recognized 70 to 80 years ago.
44,
45 It is now clear that these effects occur in humans, although the magnitude of the effect varies widely among studies.
46 Important causes of variation include the duration of protein feeding (habitual protein intake vs. meat meals or amino acid infusions), the type of protein (animal vs. vegetable or soya protein sources; essential vs. nonessential amino acids), and the filtration marker used to measure GFR (inulin vs. creatinine).
In a classic study, Pullman et al.
47 placed healthy humans on low (0.1 to 0.4 g/kg/day), medium (1.0 to 1.4 g/kg/day), and high (2.6 g/kg/day) protein diets for 2 weeks. Compared to the low protein diet, inulin clearance increased after ingestion of the medium and high protein diets by 9% and 22%, respectively. These changes were accompanied by parallel changes in renal plasma flow, indicating a hemodynamic basis for the changes in GFR. A longer period of habituation may have greater effects on GFR. Similarly, in patients with chronic malnutrition, inulin clearance was 27% to 64% lower than after repletion of nutritional status,
48,
49,
50,
51 and returned to near normal values only after 1 month of refeeding. In addition, malnourished patients had smaller kidneys, suggesting that differences in kidney function were due to structural as well as hemodynamic alterations.
48 Increases in GFR and kidney size in association with increased protein intake have been noted in diverse clinical circumstances, such as in patients receiving total parenteral nutrition and in insulin-dependent diabetic patients with poor metabolic control.
52 Some studies suggest a greater response to animal than vegetable protein in habitual diets as well as in response to protein loads.
53,
54,
55 A recent study assessing the impact of sustained high protein feeding demonstrated an increase in GFR in young subjects (24 ± 1 years old), but actually a small decrease in GFR in older subjects (70 ± 2 years old).
56After a meat meal, GFR, renal plasma flow, and splanchnic blood flow rise within an hour and remain elevated for several hours.
57 In humans, the increment in inulin clearance is about 10%,
58,
59 and appears to be less than the increment in creatinine clearance.
46 Nonessential amino acids are more potent than essential amino acids in inducing the postprandial rise in GFR, and branched-chain amino acids appear to have little or no effect.
It had been proposed that protein-induced hyperfiltration represents “renal reserve capacity,” which is lost prior to the reduction in baseline GFR associated with kidney disease.
60 However, it has now been shown conclusively that changes in GFR occur in response to changes in habitual protein intake or meat meals in patients with kidney disease and reduced GFR.
59,
60,
61,
62,
63 This is consistent
with studies in animals with experimental kidney diseases, which show that changes in protein intake further modulate the determinants of single-nephron GFR. In particular, a high protein diet raises the already increased glomerular plasma flow and transcapillary hydrostatic pressure gradient.
64,
65 Thus, protein-induced hyperfiltration augments the hyperperfusion and hyperfiltration of chronic kidney disease.
Diurnal Variation. A normal diurnal variation in filtration rate occurs, with 10% higher values occurring in the afternoon than in the middle of the night.
66 In large part, the diurnal variation is thought to be related to variation in protein intake during the day.
16,
60 Possibly, diurnal variation may also be related to transient reductions in GFR associated with exercise. Indeed, a decrease of 40% or more is seen with severe exertion.
16,
67,
68 However, diurnal variation is also observed in quadriplegics,
69 arguing against physical activity as the sole cause of diurnal variation. Possibly, diurnal variation may also reflect variation in hydration. GFR increases with overhydration and decreases with water restriction. However, the changes are small except when gross disturbances in fluid balance occur.
Antihypertensive Therapy. As a result of powerful mechanisms for autoregulation of renal hemodynamics (
Chapter 3), the level of GFR remains relatively constant throughout a wide range of blood pressure. Nonetheless, antihypertensive therapy can be associated with reductions in GFR, due, in part, to the effect of lowering blood pressure and, in part, to specific effects of classes of antihypertensive agents. Indeed, marked reduction in GFR can complicate treatment in patients with severe hypertension and acute or chronic kidney disease,
70 which is an effect thought to be due to the loss or reset of autoregulation due to sclerosis of the renal vasculature from hypertensive injury.
71 In normal individuals and in patients with kidney disease, GFR is transiently reduced by a variety of antihypertensive agents, including diuretics, beta-blockers, central alpha-2 agonists, and peripheral alpha blockers.
72 In contrast, angiotensin-converting enzyme (ACE) inhibitors, angiotensin receptor blockers (ARBs), calcium channel blockers, and directly acting vasodilators do not regularly lower GFR in healthy subjects. A large study in patients with chronic kidney disease and well-controlled hypertension showed persistent small (less than 5 mL per minute), but significant, reductions in GFR associated with the use of ACE inhibitors as well as diuretics and beta-blockers.
73 In addition, after controlling for the effect of these classes of antihypertensive agents, a small effect of lowering blood pressure remained. Because the effects of the various classes of medications and of lowering blood pressure appear to be independent, a clinically significant reduction in GFR could occur in patients with chronic kidney disease undergoing treatment with multiple antihypertensive agents.
Measurement of the Glomerular Filtration Rate
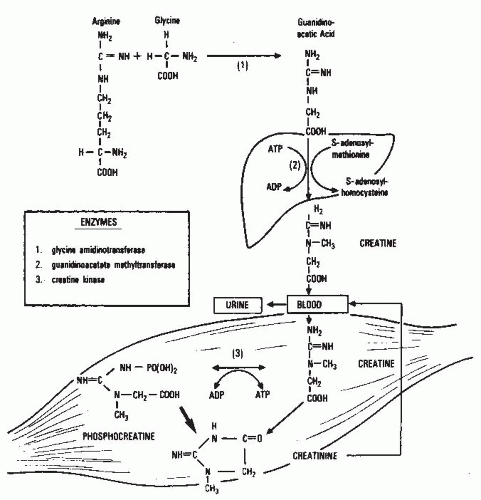
Clearance. As mentioned earlier, the GFR is assessed from the clearance of filtration markers, substances excreted by glomerular filtration that can be used to assess the GFR. The “gold standard” for the measurement of GFR is the urinary clearance of inulin. The term clearance was introduced into kidney physiology by Van Slyke and his colleagues in reference to studies of the excretion of urea in 1929.
18 Two years later, Jollife and Smith extended the use of the term to the excretion of creatinine and later to the excretion of many other substances.
74 In the many decades since these pioneering studies, the concept of clearance has maintained its primacy as the cornerstone of our understanding of the measurement of glomerular filtration.
The clearance of a substance is defined as the rate at which it is cleared from the plasma per unit concentration. The clearance of substance “x” (Cx) is given in the following equation:
where Ax is the amount of x eliminated from the plasma and Px is the average plasma concentration. Hence, Cx is expressed in units of volume per time. The value for clearance does not represent an actual volume, but a virtual volume of plasma that is completely cleared of the substance per unit of time, without reference to the route of elimination. The value for clearance is related to the efficiency of elimination: the greater the rate of elimination, the higher the clearance.
Relationship of Glomerular Filtration Rate to Urinary Clearance. For a substance that is cleared by urinary excretion, the clearance formula may be rewritten as follows:
where Ux is the urinary concentration of x and V is the urine flow rate. The term Ux × V is defined as the urinary excretion rate of x. If substance x is filtered freely across the glomerular capillary walls and excreted only by glomerular filtration, then the rate of filtration is equal to the rate of urinary excretion:
where the term GFR × P
x is defined as the filtered load of x. By substitution into
Equation 9.2:
Hence, substance x would be defined as an “ideal filtration marker” whose urinary clearance could be used to measure GFR.
However, if substance x is also reabsorbed or secreted by the renal tubules, then the following equations apply:
where TRx and TSx are the rates of tubular reabsorption and secretion of x, respectively, and TRx /Px and TSx /Px are the clearances of substance x due to reabsorption (CTRx) and secretion (CTSx), respectively. In this case, the rate of urinary excretion (Ux × V) does not equal the filtered load (GFR × Px), and clearance does not equal GFR. Therefore, the value for urinary clearance of x (Cx) is determined not only by the rate of glomerular filtration, but also by the mechanism of excretion by the kidney. For substances that are filtered and secreted, clearance exceeds GFR, and for substances that are filtered and reabsorbed, clearance is less than GFR.
Inulin Clearance. The requirements for an ideal filtration marker, as outlined by Smith,
15 include the following:
1. It is freely filtered at the glomerulus. It passes from the glomerular capillary blood into Bowman’s space unhindered by its size, charge, or binding to plasma proteins.
2. It is not altered during its passage through the nephron. It is not reabsorbed, secreted, synthesized, or metabolized by the tubules.
3. It is physiologically inert and does not alter the function of the kidney.
Inulin, a 5,200-dalton, inert, uncharged polymer of fructose, meets these criteria, and it remains the standard for experimental and clinical measurement of GFR.
15,
75,
76 The conclusion that inulin is freely filtered and is neither secreted nor reabsorbed in the normal kidney was originally based on indirect evidence, but a large body of direct micropuncture observations have verified this assumption.
11,
77,
78,
79,
80 Similar evidence is not available, however, in all experimental kidney diseases. For example, in several models of acute kidney failure with extensive tubular basement membrane damage, leakage of inulin across the tubules is readily demonstrated.
81,
82 In such situations, of course, the urinary excretion of inulin is less than the filtered load, and inulin clearance is less than GFR.
Although the measurement of inulin clearance is a highly accurate and reproducible means of estimating GFR, there are several disadvantages that make it impractical for clinical use. First, the classical method includes measurement under fasting conditions in the morning, a continuous intravenous infusion, multiple clearance periods requiring repetitive blood and urine collections over 3 hours, oral water loading to stimulate diuresis, bladder catheterization to assure complete urine collection, and careful timing of blood sampling at the midpoint of the urine collection. Period-to-period variability in GFR (intratest variation; expressed as CV) is approximately 10%. Intratest variation may reflect incomplete bladder emptying and is often used to judge the quality of a urinary clearance study.
15 However, one recent study has shown that the precision of GFR determinations is only weakly affected by intratest variability,
83 probably because averaging over several clearance periods minimizes error due to incomplete bladder emptying. In a study in normal individuals using the classical method of inulin clearance, the CV for repeated measurements within an individual (intertest CV) was 7.5%.
84 These estimates of measurement error are probably lower than would be observed in most clinical settings. Second, inulin is difficult to dissolve in aqueous solutions, difficult to measure, and is in short supply. Because of these disadvantages, clinical assessment of GFR generally utilizes other filtration markers and clearance methods.
Urinary Clearance of Endogenous Filtration Markers. In principle, the simplest alternative to inulin clearance would be the urinary clearance of an endogenous filtration marker. The advantage of this method is that clearance can be computed from urine collections and blood sampling under usual clinical conditions without the need for administration of an exogenous marker. Indeed, this method is widely used for measuring creatinine clearance, as discussed later. The most common method is to collect a 24-hour urine collection and a single serum measurement, assuming a steady state. The urine collection is performed at home. At the onset of the collection period, the patient is instructed to empty the bladder and discard the urine. During the collection period, all subsequent urine is saved. At the end of the period, the patient is asked to void completely and to add this last specimen to the urine collection. Shortly thereafter, the blood sample is obtained.
Unfortunately, the accuracy of this method is limited because neither creatinine nor any other known endogenous filtration marker meets all the criteria for an ideal filtration marker and because timed urine collections under usual clinical conditions are notoriously inaccurate. Errors in timing or completeness can result from misunderstanding by the patient or personnel of the instructions, such as omitting urine specimens during the interval or incompletely emptying the bladder at the start or end of the collection period. At first glance, it might appear that the use of short urine collection intervals, such as 1-hour, carried out under close supervision by trained personnel might overcome these difficulties. However, using a shorter collection period, the small errors due to incomplete bladder emptying would have a greater impact on the estimate of the urine volume and hence the urine flow rate. Indeed, the 1-hour technique has been largely abandoned because the extra effort and personnel required do not significantly improve the accuracy as compared to the 24-hour
clearance.
85 However, averaging the results of three to four 30-minute collection periods does significantly improve the accuracy, probably due to cancellation of errors from incomplete bladder emptying.
86A similar method can be used to compute clearance for patients who are not in a steady state balance by obtaining additional blood samples during the urine collection to estimate the average serum concentration. The most common strategies are to collect blood at the mid-point of the urine collection, or at the beginning and end of the urine collection, and to average the serum concentrations.
Alternative Clearance Methods and Exogenous Filtration Markers. All alternative clearance methods have been designed to facilitate GFR measurement; however, all have limitations that should be understood for proper interpretation.
Table 9.2 summarizes the strengths and limitations of these alternative clearance methods and markers, as well as the gold standard method.
75,
87,
88,
89
Changes to the clearance method include substitution of bolus intravenous or subcutaneous injection for a constant intravenous infusion and use of plasma clearance techniques to eliminate the need for urine collection. With a bolus injection, the pattern of decline in serum levels is more accurately modeled as an exponential rather than linear of decline.
83 In the bolus subcutaneous technique, the marker substance (e.g.,
125I-iothalamate,
51Cr-EDTA) can be given with a small dose of aqueous epinephrine to slow its release into the circulation, providing fairly constant plasma levels.
90,
91 More recently subcutaneous continuous infusions have been used.
92Plasma clearance is computed from
Equation 9.3 using either the entire area or a one-compartment or twocompartment model of the plasma disappearance plot.
93,
94,
95 There are several caveats. First, a relatively long time (3 to 5 hours) is required to accurately determine the declining plasma concentration of the marker, with longer times for people with reduced GFR. Second, filtration markers utilized for this method must meet an additional criterion of rapid equilibration with the extracellular volume, and inulin is therefore not appropriate for use.
96 Third, for some markers, simultaneous assessment of plasma and urinary clearance of a filtration marker typically yields a higher level for plasma clearance, presumably due to extrarenal excretion of the marker.
97,
98 This underestimation is more apparent at a lower GFR. Fourth, plasma clearance overestimates GFR in patients with moderate to severe edema probably because of the larger than expected volume of distribution and lower than expected plasma levels of the marker.
99Alternative exogenous markers include radioisotopelinked markers 125I-iothalamate, 51Cr-ethylene diamine tetraacetic acid (EDTA), and its analogue, 99mTc-diethylene triamine pentaacetic acid (DTPA), that can be readily and inexpensively measured using radioactive counters; and nonradioactive markers iohexol and iothalamate that can be measured by X-ray fluorescence and high performance liquid chromatography (HPLC) methods. The advantage of the latter two is the avoidance of radiation exposure; however, the assay methods are more expensive and generally performed in specialized laboratories. All other filtration markers deviate from ideal behavior. Overall, there is suggestion by some but not all studies that iothalamate clearance results in a higher GFR than inulin clearance, presumably due to secretion of iothalamate by the tubules. Other studies suggest that iohexol clearance may underestimate inulin clearance. DTPA readily dissociates from its radioactive tracer, allowing binding of the tracer to plasma proteins leading to retention of the tracer and underestimation of GFR.
GFR can also by measured by counting of a radioactive exogenous filtration marker over the kidneys and bladder. This technique can be combined with renal imaging, usually using
99mTc-DTPA, and is useful for determination of split kidney function.
88,
100 Several studies indicate poor correlation of
99mTc-DTPA dynamic renal imaging with simultaneous urinary or plasma clearance, reflecting both bias and imprecision, and lesser accuracy than estimated GFR.
101,
102,
103 Currently, magnetic resonance imaging (MRI) is being investigated for measurement of GFR. Many protocols are in use which will require consolidation before introduction into clinical practice.
104,
105Because of these limitations, all values for measured GFR contain an element of error, which differentiates them from true GFR. As such there is variability in the literature as to how each of these markers and methods compare to the gold standard method.