Fig. 21.1
Normal arterial anatomy of upper abdominal viscera. RH right hepatic artery, MH middle hepatic artery, LH left hepatic artery, PV portal vein, RGA right gastric artery, GDA gastroduodenal artery, SPDA superior pancreaticoduodenal artery, Post PDA poster branch of the superior pancreaticoduodenal artery, Ant PDA anterior branch of the superior pancreaticoduodenal artery, GEA gastoepiploic artery, IPDA inferior pancreaticoduodenal artery, SMV superior mesenteric vein, SMA superior mesenteric artery, MCV middle colic vein, MCA middle colic artery, SA splenic artery, DP dorsal pancreatic artery, LGA left gastric artery, HA hepatic artery (proper hepatic artery). (With permission from [82] © Springer 2012)
Variant Anatomy of the Hepatic Arterial Vasculature
Anatomic variations of the hepatic arterial vasculature are common, and a thorough knowledge of these anomalies is essential to preventing injury. Although Haller first published his treatise on variant hepatic arterial anatomy in 1756, a systematic analysis of hepatic arterial variations was not undertaken until 1966 when Michels described 10 anatomic variants based on 200 cadaveric dissections [6]. Following Michels, Hiatt and colleagues classified hepatic arterial variations into six types (Fig. 21.2) based on 1000 patients who underwent liver harvest for transplantation [7]. Numerous other groups have since reported on variant hepatic arterial vasculature, based on cadaveric dissections, liver harvest for transplantation, and angiographic evidence (Table 21.1) [8–11]. A hepatic arterial branch is termed replaced when it does not arise off the PHA but supplies a hemi-liver. A hepatic arterial branch is termed accessory when it supplies part of a hemi-liver in addition to an arterial branch off the PHA. The most common variations are a replaced RHA (RRHA) arising from the SMA (3–15 %), replaced LHA (RLHA) arising off the LGA (2–10 %), normal anatomy with an accessory LHA (ALHA) off the LGA (≤ 10 %), and normal anatomy with an accessory RHA (ARHA) off the SMA (≤ 7 %) (Table 21.1) [6, 9–16].
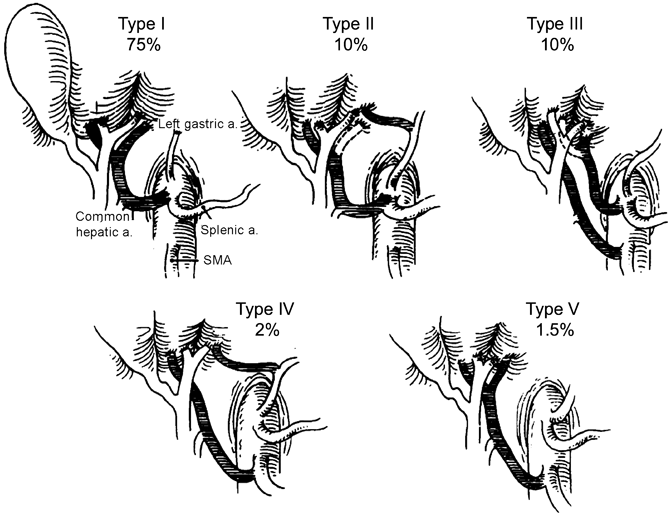

Fig. 21.2
Hiatt’s classification of hepatic arterial variations. Dotted lines indicate that the variant artery may be accessory (if branch shown by dotted line is present) or replaced (if absent). Type I: normal anatomy; Type II: replaced or accessory left hepatic artery; Type III: replaced or accessory right hepatic artery; Type IV: replaced or accessory right hepatic artery + replaced or accessory left hepatic artery; Type V: Common hepatic artery from the superior mesenteric artery; Type VI: Common hepatic artery from the aorta (not shown). (With permission from [7] © Lippincott Williams and Wilkins 1994)
Arterial anatomy | Frequency (%) |
|---|---|
Proper hepatic artery branching into right and left hepatic arteries | 52–80 |
Replaced arteries | |
Left hepatic artery from left gastric artery | 2–10 |
Right hepatic artery from superior mesenteric artery | 3–15 |
Left hepatic artery from left gastric, right hepatic from superior mesenteric artery | < 3 |
Common hepatic artery from superior mesenteric artery | 1–5 |
Common hepatic artery from left gastric artery | < 1 |
Common hepatic artery from the aorta | < 1 |
Accessory arteries | |
Left hepatic artery from left gastric artery | £ 10 |
Right hepatic artery from superior mesenteric artery | £ 7 |
Left hepatic artery from left gastric, right hepatic from superior mesenteric artery | < 1 |
Replaced and accessory arteries | |
Replaced right hepatic artery (from superior mesenteric artery), accessory left hepatic artery (from left gastric artery) | < 2 % |
Replaced left hepatic artery (from left gastric artery), accessory right hepatic artery (from superior mesenteric artery) | < 2 % |
Replaced and Accessory Right Hepatic Arteries
Aberrant RHA anatomy is the most common and surgically relevant variant. Both a RRHA and ARHA arise from the SMA, travel posterior to the pancreatic head, and enter the hepatoduodenal ligament posterolateral to the CBD. Although many anatomic courses including through the pancreatic parenchyma have been reported, including through the pancreatic parenchyma [1], a dissectable groove usually exists between these vessels and the pancreas (Table 21.1, Fig. 21.2).
Replaced Common Hepatic Artery
Celiac Artery Stenosis
Although not an anatomic variant, celiac artery stenosis (CAS) is an important vascular abnormality in HPB surgery. Blood supply through the celiac trunk is impaired, leading to retrograde flow from the SMA through the pancreaticoduodenal arcades, dorsal pancreatic artery, and arc of Buhler (an embryonic communication between the celiac and SMA observed in 2 % of population) [21]. In patients with CAS, retrograde flow through the GDA is the primary source of arterial blood to the liver and commonly manifests as an unusually large GDA or pancreatic collateral vessel. The incidence of CAS ranges from 10 to 25 % of the population [21]. The pathophysiology may be divided into the following three categories:
Extrinsic compression: It is commonly due to the median arcuate ligament, an enlarged celiac ganglion, or fibroinflammatory tissue. The median arcuate ligament joins the left and right diaphragmatic crura, contacting the aorta cephalad to the celiac trunk. However, it can pass anterior to the celiac artery in up to 25 % of individuals. Extrinsic compression is the most common cause of CAS in Asian populations (55 %) [21, 22].
Intrinsic stenosis: Intrinsic stenosis is secondary to atherosclerotic disease and is the most frequent cause of CAS in Western countries [21, 23].
Other etiologies: These include neoplastic invasion, pancreatitis, acute or chronic dissection, or intimal disruption [21].
Preoperative Radiographic Assessment
Careful radiographic assessment allows for the identification and anticipation of anatomic and pathologic factors such as variant anatomy or malignant vascular invasion that may increase susceptibility to injury, necessitate ligation, or require reconstruction. For preoperative evaluation prior to pancreas resections, the best imaging modality is a pancreas protocol CT scan, which includes contrast-enhanced thin-cut arterial and venous phase imaging through the pancreas [24]. Although direct angiography remains the gold standard for assessing vascular anatomy and is the only modality that identifies directional flow, it is rarely used as arterial phase CT angiography, with or without angiographic reconstruction has a reported accuracy of 98 % for detecting arterial anatomic variations [25, 26], and has the advantage of delineating the relationship of arteries to adjacent organs or tumor [27]. The advent of multidetector-row CT scanners has further enhanced pancreatic imaging, enabling prediction of visceral vessel involvement and resectability in 80–90 % of pancreas resections [28]. MRI typically includes arterial and portal phase imaging and is comparable with CT in predicting vascular invasion and local tumor extension. It is particularly useful when patients are intolerant to intravenous contrast agents and when greater soft-tissue contrast or visualization of the pancreatic duct and biliary tree is desired, such as while evaluating cystic pancreatic neoplasms [29]. Arterial reconstruction is also possible with MR imaging (MR angiography). Endoscopic ultrasound (EUS), an operator-dependent modality, has not been shown to be superior to CT in determining arterial involvement [30]. We do not routinely use EUS to assess resectability or vascular anatomy.
For radiographic evaluation prior to liver resection, CT scans using a triphasic protocol (non-contrast, arterial, and portal venous phase) are helpful in assessing hepatic parenchymal disorders such as steatosis, cirrhosis, lobar/segmental atrophy, as well as normal and variant hepatic anatomy. CT angiography can also be used and is a valuable tool in facilitating surgical planning and avoiding iatrogenic injury [31]. MRI/MRCP is considered by many to be superior to CT in assessing the liver and biliary tree and can be combined with MR arteriography to simultaneously assess vascular structures [29].
Preoperative Considerations
Preoperative management focuses on recognizing clinical scenarios where hepatic artery injury and subsequent arterial compromise can lead to liver and biliary ischemia/necrosis. The hepatic arteries contribute to 25 % of hepatic blood flow and 50 % of oxygen delivery [4]. Ligation of hepatic arterial branches was historically a feared complication due to the consequent risk of liver necrosis and death. These beliefs were based on very early experiences with hepatic arterial ligation—in 1933, Graham and Cannell reported a mortality rate of approximately 60 % in a review of 28 cases where the CHA, PHA, RHA, or LHA was ligated [32]. Mortality in that era, however, was heavily influenced by deficiencies in perioperative care, including anesthetic techniques, antibiotics, and transfusion medicine. In 1964, Starzl and colleagues observed in four patients that ligation of the CHA, PHA, RHA, and LHA in patients with normal liver function only resulted in mild transaminitis and not death [33]. They went on to examine all reports of hepatic artery branch ligation in patients without cirrhosis or hepatic artery aneurysms between 1933 and 1964. They concluded that ligation of any hepatic arterial branch (CHA, PHA, RHA, or LHA) in patients with normal liver function results in mild transient transaminitis and rarely leads to liver necrosis and death. Flow through the PV and arterial collaterals was sufficient to maintain hepatic oxygenation, provided factors increasing hepatic oxygen demand or decreasing PV blood flow (shock, jaundice) were absent. These seminal early observations established the safety of hepatic arterial branch ligation and served as the basis for later investigations into its mechanisms and therapeutic potential.
Following these data demonstrating its safety, Plengvanit demonstrated that ligation of the CHA, RHA, or LHA resulted in collateral formation commonly through the right inferior phrenic and subcostal arteries in addition to multiple other collateral sources, which was evident angiographically as early as 1 week after ligation [34]. Mays and Wheeler made similar observations, demonstrating collateral circulation could develop as early as 10 h after ligation of the RHA or LHA [35]. With these data and advances in perioperative care of the surgical patient, hepatic artery ligation was used to control hemorrhage in the setting of liver trauma [36, 37] and also as therapy for metastatic disease to the liver [38]. Ligation of the PHA was accompanied by a transient increase in transaminases, alkaline phosphatase, and bilirubin, confirming the earlier observations by Brittain and Starzl [33, 39]. We employ these principles of hepatic arterial branch ligation routinely in HPB surgery, particularly during placement of hepatic arterial pumps for regional chemotherapy [40, 41]. We have noted through dye injection perfusion tests performed while placing hepatic artery pumps that cross-perfusion after ligation of arterial branches occurs within minutes. These principles have also been utilized for tumors of the body/tail of the pancreas involving the celiac axis, where en-bloc resection of the celiac and CHA is performed (Appleby procedure) after confirming adequate collateral flow through the GDA [42, 43]. In summary, with respect to the risk of clinically significant liver ischemia/necrosis, ligation of hepatic arterial branch(es) is safe in patients with normal liver function, no jaundice, and hemodynamic stability, provided a single remaining hepatic arterial branch is patent (Table 21.2). Ligation of the CHA or PHA in patients with normal liver function, no jaundice, and hemodynamic stability has also been shown to be safe; however, avoiding injury is preferable and reconstruction of an injured CHA/PHA is reasonable. In the setting of liver dysfunction or jaundice, hepatic reliance on arterial supply for oxygenation is increased, possibly due to increased metabolic demand of hepatocytes and greater susceptibility to hypoxia and decreased intrahepatic portal flow due to local compression from dilated bile ducts [44–46]. Ligation of any hepatic arterial branch in these settings should be avoided as it may worsen liver dysfunction and precipitate liver failure.
Table 21.2
General principles of hepatic artery preservation
No jaundice or liver dysfunction |
Ligation of the CHA and PHA has been shown to be safe; however, attempts at reconstruction are reasonable |
Ligation of a hepatic arterial branch(es) is generally safe with one patent hepatic arterial branch |
Ligation of all hepatic arterial branches is generally not advised although historical data have shown it to be safe |
Jaundice or liver dysfunction |
Ligation of any hepatic arterial branch is not advised due to the risk of hepatic ischemia/necrosis |
Biliary anastomosis |
Ligation of either RHA/RRHA or GDA alone is safe |
Ligation of both RHA/RRHA and GDA is not advised due to the risk of anastomotic dehiscence or stricture |
A second concern with ligation of a hepatic arterial branch, primarily the RHA/RRHA, is the effect on the biliary tree. Isolated ligation of the RHA/RRHA has not been shown to increase the risk of biliary stricture formation or biliary anastomotic dehiscence, likely due to arterial cross-perfusion at the hilar plate from the LHA, and intact blood supply from the GDA [47]. However, interruption of both components of biliary blood supply (RHA and GDA) in the setting of a biliary reconstruction is associated with a risk of anastomotic dehiscence and stricture formation [48, 49].
Preoperative interventions are therefore directed toward clinical situations that may violate principles of hepatic artery preservation, thereby increasing the risk of liver and biliary complications (Table 21.2). Three preoperative techniques have been described to minimize the risk of liver ischemia when a hepatic artery branch is at risk for injury. The first and most commonly used technique is preoperative biliary drainage in jaundiced patients. Although demonstrated to increase overall perioperative complications for pancreas resections [50, 51], preoperative biliary drainage improves liver function and likely relieves pressure on the portal system from the bile ducts, thereby minimizing the risk of postoperative liver ischemia [52]. We recommend preoperative biliary drainage in patients with obstructive jaundice when a hepatic arterial branch is at risk for injury or clearly requires ligation or reconstruction (and hence risks thrombosis) during surgery (Fig. 21.3). A second technique that has been described but is less commonly used is embolization of the arterial branch to be sacrificed, to preoperatively promote development of collateral flow to the corresponding hepatic segment(s), thereby minimizing postoperative ischemia [53]. We have only occasionally used this technique in our practice. A third preoperative technique to minimize liver ischemia, used in patients with CAS and expected GDA ligation at surgery, is celiac artery stent placement (Fig. 21.3). Stenting of the celiac artery has been reported to decrease the risk of biliary/pancreatic anastomotic disruption and liver ischemia [54–57], with 80–95 % success rates [21, 58, 59]. Anticoagulation to prevent stent thrombosis and an adequate waiting period to allow for collateral development are important considerations prior to staged resection. If stenting is not possible, surgical bypass, either at the time of or prior to planned resection, is the only option.
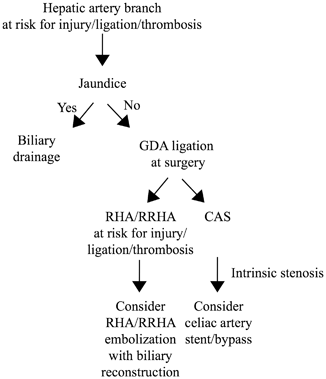

Fig. 21.3
Preoperative vascular considerations in hepatobiliary and pancreatic resections. RHA right hepatic artery, RRHA replaced right hepatic artery, CAS celiac artery stenosis, GDA gastroduodenal artery
Preoperative considerations to minimize the risk of biliary ischemia are relevant in patients with a RRHA and GDA that will be ligated or are at risk for injury or thrombosis. In this setting, embolization of the RRHA to allow for collateral flow to develop to the bile duct prior to resection and anastomosis has been reported (Fig. 21.3) [60]. However, we rarely use this technique as the RRHA can often be preserved without margin compromise, or can be reconstructed [61, 62].
Intraoperative Considerations
Meticulous dissection with complete exposure and identification of structures prior to division is essential to prevent inadvertent hepatic artery injury. The CHA can be identified by its relationship to the hepatic artery lymph node (HALN), located at the superior border of the pancreas, medial to structures in the HD ligament. The HALN abuts the superior wall of the CHA, just proximal to the GDA origin and careful removal exposes the CHA near the GDA origin. The CHA can be mistaken for the RHA, the LHA, or even the splenic artery and inadvertently ligated, underscoring the need for complete exposure and identification as well as test clamping prior to division of any structure. Injury to the CHA and PHA can be difficult to successfully suture repair primarily, although this has been described [63]. Reconstruction options include primary anastomosis, transposition of native arteries (splenic artery, right gastroepiploic artery, GDA) [64–68], or interposition grafts with autologous tissue such as the gonadal vein [69]. Vascular reconstruction of the hepatic artery is technically challenging and not commonly performed by HPB surgeons; hence, assistance from a vascular or transplant surgeon can be helpful and sought out if necessary.
Specific Intraoperative Considerations
Pancreaticoduodenectomy (PD)
Replaced/Accessory Right Hepatic Artery
A RRHA/ARHA is in close proximity to the bile duct and head of the pancreas (and therefore close to tumors in the head of the pancreas) in the posterolateral space of the hepatoduodenal ligament and is therefore at risk for injury during a PD. If the patient is jaundiced, preoperative biliary drainage is indicated (Fig. 21.3). Meticulous dissection during mobilization of the CBD, duodenum, and pancreatic head commonly allows preservation of a RRHA/ARHA, without compromise of margin status or outcomes [61, 62]. Altering the operative approach has also been described as a technique to minimize the risk of injury. The most common approach to a PD is through an anterior approach, dissecting the head and uncinate process off the PV, followed by dissection along the SMA. Although this may still be safely feasible, a posterior or “artery first” approach should be considered when a RRHA/ARHA is noted. A posterior approach allows for early assessment of resectability, SMA identification, and proximal control [70–74]. If intraoperative injury or involvement by tumor necessitates ligation of a RRHA, reconstruction is recommended to prevent bilio-enteric anastomotic and hepatic ischemia as the GDA is commonly ligated in a PD. Reconstruction of an ARHA is advised in jaundiced patients to prevent liver necrosis, as discussed earlier (Table 21.2). Injury to the RRHA/ARHA can be repaired by primary anastomosis, venous or prosthetic interposition, or reconstruction using a ligated GDA stump [65, 75–77].
< div class='tao-gold-member'>
Only gold members can continue reading. Log In or Register to continue
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree







