Fig. 9.1
Flexible laparoscopic ultrasound probe (From Hitachi-Aloka, Wallingford, CT)
Recently, a robotic transducer, the ProART (BK Medical Herlev, Denmark), with Doppler capability was developed. It has a unique fin over the array, which can be grasped by robotic instruments. Yakoubi et al. demonstrated the feasibility of this novel probe in an animal model [13]. Similarly, another novel fully articulated transducer has been developed to reach complex angles that are difficult with traditional flexible LUS probes (Hitachi-Aloka, Tokyo, Japan) [14]. This novel drop-in transducer probe has a 33-mm width linear array with a frequency rate of 4–13 MHz. Robotic instruments can also easily handle it, and the surgeon can remotely control its movement with greater precision and dexterity (Fig. 9.2). Additionally, micro-ultrasound probes have been made for better handling with robotic instruments (Fig. 9.3). The da Vinci surgeon console has a software imaging program called TilePro (Intuitive Surgical, Sunnyvale, CA) that allows up to two adjunctive imaging inputs to be viewed by the surgeon simultaneously with the regular 3D camera field view. Figure 9.4 shows real-time ultrasound visualization in the surgeon console during robotic nephron sparing surgery. While the image from the robotic camera is seen on the main screen, the ultrasound image is visible simultaneously in the lower left-hand corner.

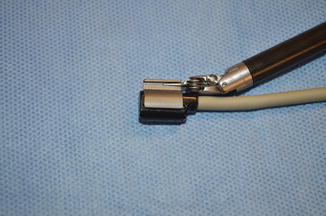
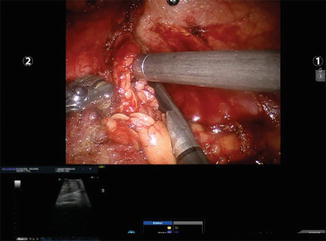

Fig. 9.2
Robotic flexible ultrasound probe (Courtesy of Hitachi-Aloka, Wallingford, CT)

Fig. 9.3
Robotic micro-ultrasound probe (From Hitachi-Aloka)

Fig. 9.4
Real-time ultrasound visualization in the surgeon console. A renal mass is being identified
Fundamentals of Ultrasound Doppler Imaging/Sensing
The basis of Doppler ultrasound sensing is that as an object emitting sound moves, the wavelength of the perceived sound is compressed and its frequency is increased in the forward direction. In the receding direction, the perceived wavelength would be expanded and frequency decreased. Doppler ultrasound probes have the ability to detect velocity or flow of material based on this measured increase or decrease of sound frequency. The delay in wave sensing between the primary and secondary reflected waves depends on the depth and the speed of the sound waves in various tissues [15]. Several LUS probes today have Doppler ultrasound capability, which allows for differentiation of blood vessels or highly vascularized masses compared to normal parenchymal tissue.
Recently, a sterile, disposable laparoscopic drop-in Doppler ultrasound (LDU) probe has been introduced for robotic procedures (Fig. 9.5) (VTI Vascular Technology Inc., Nashua, NH). The system includes a Doppler transceiver (8 or 20 MHz) with flexible disposable probes that emit ultrasound waves and sense reflected echo from blood flow. The 8 MHz is used to detect larger vessels such as the renal artery and vein. The 20 MHz probe is used to detect smaller vessels (1 mm or less) for microsurgical procedures (such as the testicular artery). This system provides an auditory pulse signal to identify blood flow allowing clear differentiation of arteries and veins [16].
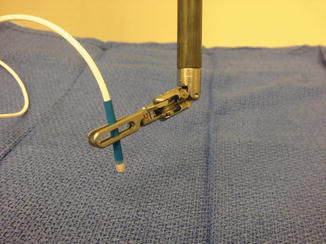

Fig. 9.5
Disposable audible micro-Doppler probe (Courtesy of Vascular Technology Inc., Nashua, NH)
Intraoperative Doppler Ultrasound During Ablative and Reconstructive Robotic Renal Surgeries
Both extirpative and reconstructive robotic renal procedures are performed routinely with the da Vinci robotic platform such as radical and partial nephrectomy, nephroureterectomy, living-donor nephrectomy, pyeloplasty, and renovascular surgery [17]. Surgical maneuvers with robotic instruments around the renal hilum can in some cases be intimidating because of the lack of tactile feedback, scarring from prior surgeries and due to variations in renal vascular anatomy. A preoperative imaging study using helical CT angiography looked at 102 living donors. It revealed the presence of multiple renal arteries (31 % on the left, 20 % on the right) and multiple renal veins (5 % on the left, 20 % on the right) [18]. Although advanced preoperative imaging techniques such as multi-detector helical CT or MR-angiography are reasonably accurate in showing renal vascular anatomy, accessory vessels can be missed by imaging techniques [19, 20]. Even though this occurs in a small number patients, it can lead to surgical complexity and result in open conversion due to bleeding from unexpected vessel trauma [21]. Additionally, patient positioning, pneumoperitoneum, and intraoperative mobilization during robotic renal surgery can change the anatomical relationship between the renal hilum and other landmarks compared to preoperative imaging studies [22]. Recently, an analysis of 886 robotic partial nephrectomy procedures revealed that perioperative hemorrhage occurred in 0.5 % of the patients (two due to renal vein injury and two due to a missed unclamped renal artery) [23]. Lee et al. also described iatrogenic injury of the posterior segmental branch of renal artery during robot-assisted partial nephrectomy [8]. Unfortunately, these injuries can result in renal parenchymal loss, excessive blood loss, and longer operative times. Robotic pyeloplasty procedures can become a challenge due to obesity, a large floppy liver, redundant colon, crossing vessels, large calculi, and previous surgery [24].
For all the reasons mentioned above, IODU provides surgeons with an adjunctive tool to assist in identifying vessels and tissue perfusion during complex and challenging robotic renal procedures.
Intraoperative Audible Doppler and Ultrasound Imaging During Robotic Partial Nephrectomy
The incidence of kidney cancer has globally increased possibly due to increased prevalence of risk factors and common utilization of imaging techniques [25]. There has also been a shift to increased detection of smaller and Stage 1 tumors from more advanced tumors [26]. These smaller, earlier stage kidney tumors are better suited for minimally invasive approaches such as partial nephrectomy [27]. Today, with the advantages of the robotic platform, the number of robotic partial nephrectomy (RPN) procedures is progressively increasing [28].
The flexible laparoscopic ultrasound probe is very helpful in RPN and is used to demarcate tumor margins after Gerota’s fascia is opened [29]. It is particularly helpful for tumors that have more of an intraparenchymal component. A previous study illustrated that surgeons were more likely to use IODU when the tumor was more intraparenchymal [30].
Kaczmarek et al. reported preliminary outcomes of the drop-in robotic ultrasound probe (Hitachi-Aloka, Tokyo, Japan) for tumor identification in RPN [14]. Ninety-six percent of the tumors were endophytic with a mean size of 2.7 cm. They successfully completed 22 RPN with negative surgical margins using the robotic ultrasound probe without any intraoperative complications. They also reported that the robotic ultrasound probe could be articulated by the robotic surgeon to maintain perpendicular contact between the probe and kidney surface even in difficult angles [14].
The same drop-in probe was also compared with a four-directional flexible laparoscopic ultrasound probe during 150 RPN procedures [31]. There was no statistically significant difference in perioperative parameters and functional and oncologic outcomes. However, the authors found that the robotic drop-in probe could provide surgeon autonomy when an experienced surgical assistant is not available [31].
Hyams et al. reported the use of a simple 5 mm rigid disposable Doppler probe that produced audible signals for renal hilar vessel identification [22]. This probe was able to identify aberrant or accessory vessels that were not seen in preoperative imaging studies. The probe also confirmed ischemia or no flow in the main renal vessels during clamping and restoration of flow after unclamping during RPN [22]. The study demonstrated that the mean Doppler scanning duration for the renal hilum was 1.5 min and the mean renal hilar dissection duration was 7.9 min (26 RPN cases). They also reported that surgical management was altered with the Doppler evaluation in five patients (19 %) either by repositioning of the hilar clamp or additional selective arterial clamping [32]. Sethi et al. evaluated the disposable Doppler ultrasound in 20 laparoscopic partial nephrectomy patients. And they concluded that the system was easy to use without any formal training [33]. They also reported that the assessment of the renal hilum with the disposable Doppler probe provided a “road map” in patients who have anatomic distortion due to infection and previous surgery. It was also been pointed out that standard laparoscopic ultrasound probes are side firing and required careful interpretation; however, the small end firing tip of the audible Doppler probe provided very precise vascular localization and was easier to interpret. The study suggested that the identification of renal hilar vascular anatomy was easier and faster with the disposable audible Doppler probe compared to standard laparoscopic ultrasound imaging probes [33].
Standard Doppler ultrasound appears to be more sensitive in confirming no flow or ischemia in the renal hilar vessels during clamping (100 % vs. 85 %) when compared to the disposable audible Doppler ultrasound during 20 laparoscopic partial nephrectomy cases [16]. This study also showed that the disposable audible Doppler probe was faster and easier to use: The mean duration for hilar blood flow assessment was 68.6 s with the standard laparoscopic Doppler ultrasound and 44.5 s with the disposable audible Doppler probe. The disposable audible probe was able to provide adequate renal vascular and parenchymal sensing from a range of different angles [16].
Hyams et al. compared the hilar dissection operative duration with and without the disposable Doppler probe in 27 and 26 RPN procedures, respectively [34]. They found that hilar dissection time was significantly less with the use of the disposable Doppler probe (7.2 min vs. 11 min, P < 0.05). This study also further illustrated that the use of the probe led to a change in the surgical management of seven patients (26 %) during 27 RPN procedures: either by detecting accessory vessels or identifying incomplete clamping.
The key value of using such adjunctive technology in RPN is if it really makes a difference in surgical management. Table 9.1 summarizes a review of recent studies that have shown an advantage in the use of intraoperative Doppler and ultrasound imaging in terms of altering surgical management during partial nephrectomy procedures. The use of this technology has impacted surgical management in these cases from 10 to 38 % of the time (Table 9.1). The audible Doppler probe cannot evaluate tumor morphology, margins, and depth, and therefore in some cases, the standard Doppler ultrasound probe may provide more functionality [22].
Table 9.1
Listing of studies showing the impact of adjunctive intraoperative Doppler ultrasound in altering surgical management in partial nephrectomy
Study | Surgical technique | Number of partial nephrectomy cases | Number of cases in which intraoperative Doppler altered surgical technique |
|---|---|---|---|
Hyams et al. [22] | Laparoscopic | 8 | 3 (38 %) |
Mues et al. [16] | Laparoscopic | 20 | 2 (10 %) |
Perlmutter et al. [32] | Laparoscopic | 26 | 5 (19 %) |
Hyams et al. [34] | Robotic | 27 | 7 (26 %) |
Intraoperative Audible Doppler and Ultrasound Imaging During Robotic Radical Nephrectomy and Nephroureterectomy
A recent study on 855 patients who underwent angiographic evaluation to assess renal artery anatomy and its variations illustrated that accessory renal arteries were present in 16 % of cases on the right side and 13 % of cases on the left side. This variation in renal arterial anatomy may allow adjunctive intraoperative arterial mapping tools helpful for surgeons. Hyams et al. have illustrated that the audible Doppler probe can precisely identify renal arterial anatomy or any variations and help surgeons during real surgery [22]. The disposable audible Doppler probe can also be used to confirm the absence of aberrant arterial flow before ligating the renal vein in nephrectomy cases. The use of Doppler mapping did not seem to add much time to the total operative time and was relatively easy to use. Perlmutter et al. found that the mean Doppler imaging time was 1.8 min and the mean hilar dissection time was 10.4 min during 16 laparoscopic renal cases (11 radical nephrectomy and 5 nephroureterectomy cases) [32]. The utility of such mapping may also have benefits when training surgeons to perform such procedures and to build their confidence in understanding renal arterial anatomy and its possible variations.
Intraoperative ultrasound can be employed during robotic radical nephrectomy (RRN) procedures especially for tumors that extend into the vena cava. Abaza et al. described the utilization of the flexible laparoscopic ultrasound probe to identify the extent of the thrombus within vena cava [35]. A similar technique is reported by Lee et al. to visualize the tumor thrombus during RRN and vena caval tumor thrombectomy [36].
Intraoperative Audible Doppler and Ultrasound Imaging During Robotic Living-Donor Nephrectomy
The safety and efficacy of robotic donor nephrectomy (RDN) has been demonstrated even in living donors who have multiple renal arteries [37]. Laparoscopic donor nephrectomy can be performed either by hand-assisted or robotic-assisted techniques [38, 39].
Sethi et al. have reported the feasibility of the use of a disposable audible Doppler ultrasound probe during eight pure laparoscopic donor nephrectomy procedures [33]. In their study, they demonstrated that audible Doppler mapping was able to accurately detect all renal units that had two renal arteries at the time of donor nephrectomy. Thus, it was suggested that intraoperative Doppler mapping be used to accurately characterize the hilar renal anatomy and improve future graft function [33].
Van der Meijden et al. have shown that tactile feedback, especially in cases where palpation of aberrant vessels may help, may reduce surgical errors and potentially increase patient safety [40]. RDN is such a case where the use of intraoperative Doppler mapping may have a direct impact in reducing both donor and recipient morbidity by providing surgeons an accurate anatomical assessment of renal hilar vasculature.
Intraoperative Audible Doppler and Ultrasound Imaging During Robotic Assisted Pyeloplasty
Robotic-assisted pyeloplasty (RAP) is a viable and increasingly utilized alternative to conventional laparoscopic pyeloplasty [41, 42]. Previous imaging studies have shown that approximately 45 % of patients who have ureteropelvic junction (UPJ) obstruction had a crossing vessel as the cause of the obstruction [43, 44]. Intraoperative surgical complication rates during RAP are low [45–48]. However, unrecognized crossing vessels can be injured during the dissection of the UPJ and lead to intraoperative bleeding [24]. The use of intraoperative Doppler mapping may reduce this potential risk of inadvertent vascular injury.
Hyams et al. illustrated that the utilization of intraoperative Doppler mapping during RAP altered the surgical course of treatment in one out of three cases (33 %) since a crossing vessel was identified using the Doppler that had been missed on the preoperative imaging studies [22]. This study also found that use of the Doppler helped to reduce operative duration by aiding the surgeon in clearly identifying the renal vascular anatomy during UPJ dissection. Perlmutter et al. have also demonstrated that the mean Doppler mapping duration to identify crossing vessels during eight RAP cases was only 2.6 min and that this led to an approximately 15 min reduction in overall operative duration by allowing for an easier dissection of the UPJ [32].
< div class='tao-gold-member'>
Only gold members can continue reading. Log In or Register to continue
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree








