Fig. 11.1
(a) Sagittal view of the prostatic apex. In this view near the midline of the prostate, the structures can be challenging to distinguish. (b) Sagittal view of the lateral aspect of the prostate. When one rolls the ultrasound laterally away from the midline, often these three structures [prostatic apex, rectum, and sphincter] are more readily distinguishable
When one wishes to better define the apex of the prostate, the urinary sphincter, and the rectal wall, it is best to angle the transducer slightly away from the midline toward the lateral aspect (Fig. 11.1b). In this case, the anatomy is well delineated as the patient has an abundance of hyperechoic fat surrounding these structures. The prostatic apex is denoted by the arrow. Also note the junction between the prostate and seminal vesicle (curved arrow).
Defining the Course of the Urethra
It is interesting to note the variations in the course of the urethra among men. In general, the urethra tends to be located within the middle of the prostate from a horizontal or transverse perspective; however, in the anterior-posterior view, there is much variation. The urethra is apt to have an “S-shaped” curve that can deviate to various degrees along the Y-axis (Fig. 11.2a). For this reason, it is better to keep all cryoprobes sufficiently away from the urethra in order to avoid inadvertent injury (Fig. 11.2b). Many cryosurgeons avoid a segment of tissue both anterior and posterior to the urethra referred to as the “urethral zone.” Probes should rarely be placed in this area; rather, it is better to move the probes laterally outside the urethral zone to avoid inadvertent injury or a subsequent prostatic urethral slough.
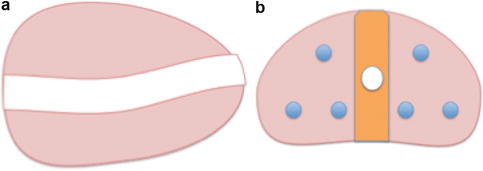

Fig. 11.2
(a) Drawing depicting the natural “S-shaped” curve of the urethra when viewed sagittally. There is much variation in the course of the urethra among men. (b) Transverse view of the prostate depicting the “urethral zone” as highlighted in orange. It is best to keep the cryotherapy probes outside of the urethral zone to avoid injury. In this figure, the cryoprobes (blue circles) are placed around, but not within, the urethral zone
Figure 11.3a displays a sagittal view of the prostate, with the posterior urethra at the base of the prostate in its normal expected position. However, toward the prostatic apex, the urethra courses anteriorly significantly. This observation is important as treatment probes need to stay away from the course of the urethra. Similarly, on the transverse view, note how anteriorly displaced the urethra is in the mid-prostate (Fig. 11.3b).
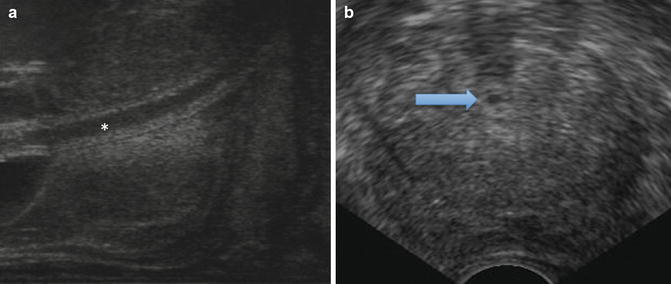

Fig. 11.3
(a) Sagittal, midline view of the prostate with an indwelling Foley catheter (white asterisk) within the urethra. (b) Transverse view of the mid-prostate with the urethra displaced anteriorly (blue arrow)
Setting the Amount of Compression by the Transrectal Ultrasound Probe
Before one begins to apply energy to treat the prostate, one needs to ensure that Denonvilliers space is sufficiently wide and that there is not undue compression of the prostate by the ultrasound probe. Figure 11.4a shows a transverse view of the prostate. Note that there is too much compression by the transrectal ultrasound probe on the posterior capsule of the prostate causing the lateral wings to appear to deviate further posteriorly (the “Mickey Mouse ears” sign). Actually, the exaggerated compression in the midline (large arrow) is causing the left and right segments of the prostate (small arrows) to move offscreen. Also note that the Foley catheter (white asterisk) is marked by two hyperechoic lines in addition to some reverberations and shadowing seen anteriorly. The urethra has a slight deviation to the patient’s right (left of the screen). Finally, the separation between the transition zone and the peripheral zone is readily apparent (curved arrow).
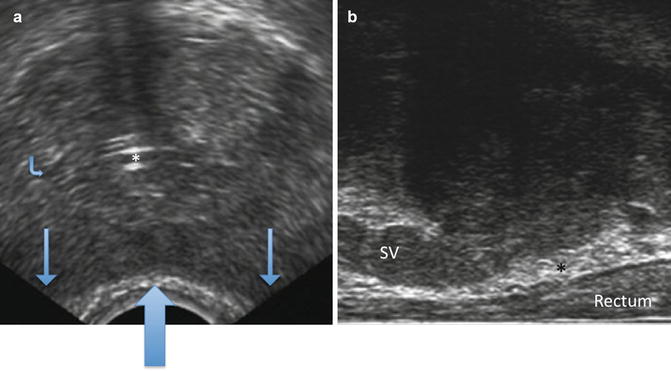

Fig. 11.4
(a) Transverse view of the mid-prostate. (b) Sagittal view of the prostate
In Fig. 11.4b, the transrectal ultrasound probe has been dropped further posteriorly so that there is not problematic compression of the transrectal ultrasound on the posterior prostate as evidenced by a very thick, non-compressed isoechoic rectal wall and a generous fat-laden (hyperechoic) Denonvilliers space (black asterisk). Also note that the seminal vesicle (SV) is inserting midway along the sagittal length of the prostate.
Ultrasound Identification of the Seminal Vesicles
The insertion of the seminal vesicles and associated ducts along the sagittal length of the prostate can vary between patients. In Fig. 11.4b, note that this patient’s seminal vesicle is easily identified as it is surrounded by hyperechoic fat within Denonvilliers fascia and also between the anterior seminal vesicle and the posterior wall of the prostate. This seminal vesicle inserts quite distally toward the apex. It is important to appreciate this on a sagittal view as when one is treating the prostate with cryotherapy and the ice is approaching the rectum as viewed sagittally, one needs to know where the edge of the prostate capsule is compared to the seminal vesicle. In this particular patient, cryotherapy would freeze a significant portion of the seminal vesicle length that is anterior to the rectal wall.
Figure 11.5a shows a transverse view of the prostate and the seminal vesicles taken between the mid-prostate and the base. The seminal vesicles are relatively hypoechoic as these are fluid-filled structures, which contrast the isoechoic nature of the dense parenchyma comprising the prostate. The posterior prostate and seminal vesicles are separated by a hyperechoic rim of adipose tissue within Denonvilliers fascia. Note the characteristic ultrasound appearance of the Foley within the urethra represented as two hyperechoic horizontal lines and the resultant shadowing in the far field. Again, it is important to appreciate these subtleties as when one is dropping the ice front posteriorly toward the rectal wall, one needs to be able to distinguish where the prostate ends and any tissue that might represent the seminal vesicles.
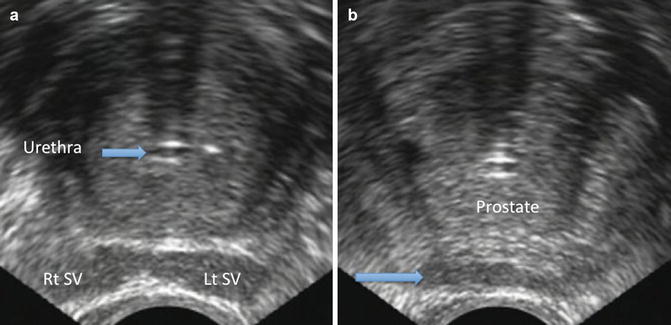

Fig. 11.5
(a) Transverse view of the prostate showing relationship to left and right seminal vesicles and the catheterized urethra. (b) Transverse view of the prostate still shows a segment of the seminal vesicles and associated ducts posteriorly, as indicated at the level of the blue arrow
Figure 11.5b shows a transverse ultrasound section of the prostate taken further toward the apex in the same patient. In this transverse view of the mid-prostate, one may initially think that they see the posterior capsule; however, the hypoechoic structures (arrow) sitting just anterior to the rectum are remnants of the seminal vesicles and associated ducts. Note also in this patient that the urethra is perfectly midline.
Localizing the Neurovascular Bundles
For some patients who are interested in attempting to preserve erectile function, localizing the neurovascular bundles before treatment is necessary. The neurovascular bundles can usually be found at 5 and 7 o’clock on the posterolateral surfaces of the prostate bilaterally. Color Doppler, or alternatively power Doppler, can be utilized to pinpoint these important structures.
Figure 11.6 demonstrates the right neurovascular bundle as highlighted with power Doppler. Also note that the hypoechoic area just anterior to the rectal wall represents the most distal aspects of the seminal vesicles and associated ducts, not the prostate.
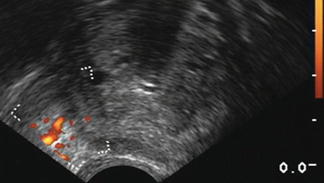

Fig. 11.6
Transverse view of the prostate and the right neurovascular bundle using power Doppler. Note the characteristic appearance of the urethra containing a catheter and the acoustical shadowing seen beyond it
Incomplete Ablation of the Prostate
The premise of prostate cryoablation is that all parenchymal tissue will be effectively treated within the intended zone of ablation. A number of reasons for incomplete ablation, often inadvertent, of segments of the prostate are possible. Clinically, inadequate ablation can be identified by persistent prostate-specific antigen (PSA) after treatment (biochemical persistence) or by a positive post-treatment biopsy demonstrating malignancy. Since prostate cryotherapy is not intended to ablate the entire gland, some degree of PSA elevation after therapy is expected. For example, the urethral warming catheter is utilized in an attempt to preserve the urethra, thereby preventing urethral slough. Prostate tissue circumferentially located a few millimeters beyond the caliber of the urethra is likely not frozen to the required temperature that causes cell death. It is rare for primary prostate cancers to grow in areas exquisitely close to the urethra, but on occasion, these tumors can be identified by surgical pathology following a salvage radical prostatectomy after radiation failure. However, in the primary setting, preserving a small rim of periurethral tissue is likely clinically insignificant. The first step to understanding the concept of incomplete ablation of segments of the prostate revolves around understanding the particular cryotherapy technology that one is using. The ice ball grows radially from the probe outward. The isotherms, or temperature gradients within the ice region, are not uniform (Fig. 11.7). For example, the outer edge of the ice typically measures 0 °C and is not thought to be lethal. Lethal temperatures beginning with the minus 20 °C isotherm are encountered approximately 3–4 mm within the edge of the ice ball. As one proceeds deeper within the ice ball, temperatures within the minus 40° Celsius isotherm are encountered. These temperatures are usually cold enough to kill most cancers [3].
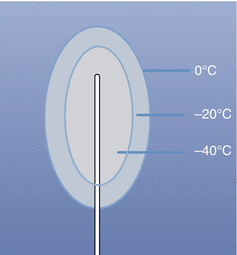

Fig. 11.7
Schematic depicting the cryoprobe and corresponding isotherms. The ice generated exists within a temperature gradient based on distance from the source. The shaded colors depict temperature approximations within that particular isotherm
In order to eradicate the target parenchyma, the probes need to be placed in such a manner that the ice fields overlap without pockets of tissue that are warmer than minus 20 °C. Thus the operator must be familiar with the particular manufacturer’s cryotherapy products and their associated isotherms. Figure 11.8a shows a schematic of a transverse view of the prostate with good probe placement allowing for sufficient overlap of ice fields. However, as is demonstrated in Fig. 11.8b, there is a variable free zone (warm pocket) between the proximal and distal ice fields posteriorly when viewed in the sagittal dimension. Therefore, one must be vigilant when evaluating proper probe placement in both the transverse and sagittal views.
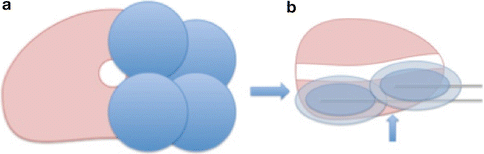

Fig. 11.8
(a) Transverse schematic showing correct cryoprobe placement. Each blue round circle represents an ice ball. Each of these ice balls overlaps to some degree, thereby obviating any warm areas of incompletely treated tissue. (b) Sagittal view of nonoverlapping ice fields. In this case, there are two areas indicated by the arrows that have incompletely treated tissue
Another potential cause for an untreated area of parenchyma is due to skewed probe placement (Fig. 11.9a). All cryoprobes should be placed in proper horizontal position through the stepper. As is illustrated in Fig. 11.9a, the anterior-most probe is placed in a non-horizontal fashion. One can see that as the probe gets farther away from the other probes from the apex of the prostate, the angle itself widens (Fig. 11.9a. b).
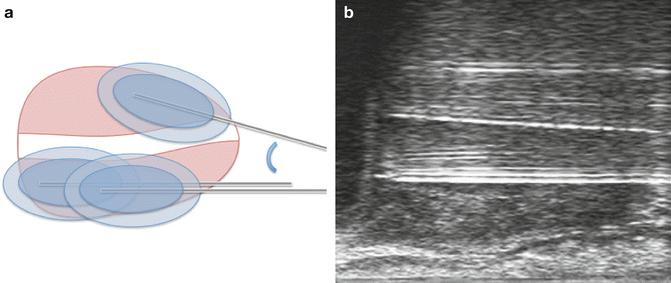

Fig. 11.9
(a) Schematic of skewed probe placement. Note that the initial takeoff angle between the first and second row probes is great, but as the probe moves from apex to base of the prostate, the distance between consecutive probes increases. (b) Sagittal ultrasound view demonstrating the middle probe has an angulation anteriorly. All probes should be placed horizontally and in parallel with adjacent probes
Men having a median lobe may account for persistent PSA elevation following cryoablation. In general, it is unusual for cancer to occupy the median lobe, but it is quite common for the median lobe not to be completely treated, depending upon its size. Freezing the median lobe, although it would reduce the PSA further, may be risky due to the proximity of the median lobe to the ureteral orifices. Finally, one needs to be sure that the posterior-most segment of the prostate, including the capsule, is sufficiently treated with lethal temperatures. As previously stated, the ice ball needs to run 3–4 mm beyond the posterior capsule to achieve this. In order to prevent inadvertent injury to the rectum, the rectal wall needs to be dropped further posteriorly away from the ice field. This can be accomplished either by reducing the anterior pressure on the transrectal ultrasound itself or, alternatively, by injecting saline into Denonvilliers space. Either of these maneuvers will help to widen Denonvilliers space and reduce compression on the rectal wall.
Another possible area of incompletely treated tissue is the prostatic apex. This may be due to the physician’s reluctance to aggressively freeze this region due to the surrounding rectal wall and urinary sphincter. Figure 11.10a depicts a schematic of the ice ball covering the posterior apex of the prostate and avoiding the urinary sphincter. Note that the “rectal hump” toward the apex of the prostate has a gentle curve upward that matches the natural anterior curve of the ice ball (Fig. 11.10b). Some physicians believe that by utilizing a minimum of two thermocouples placed at the urinary sphincter and Denonvilliers fascia allows safe monitoring of the temperature while freezing. In addition, paying attention to proper probe placement, ice field coverage, and adjacent structures will lead to better oncologic and functional results.
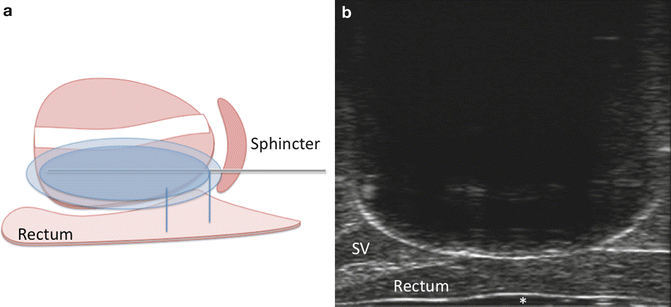

Fig. 11.10
(a) Schematic sagittal view of ice ball covering the apex of the prostate. Despite the proximity of the rectum and the urinary sphincter, it is feasible to achieve sufficiently cold and lethal temperatures at the apex while avoiding collateral damage to the sphincter and rectum. (b) Sagittal view of the ice ball upon completion of freezing. The hyperechoic rim of the ice ball can be easily seen resting upon the rectal wall. Note that (1) the entire prostate is covered in ice, (2) there is minimal compression of the rectal wall and it remains quite thick, and (3) a portion of the seminal vesicle (SV) base often is incorporated in the ice field. The asterisk (*) denotes the ultrasound jelly within the condom sheath [the white hyperechoic line] surrounding the transrectal ultrasound probe
Primary Prostate Cryoablation
When positioning the patient for cryotherapy, it is important that he be placed in a true lithotomy position. In this respect, the lower legs should be parallel to the floor and be approximately at a right angle to the upper legs. This position should minimize the chance of pubic arch interference, sometimes encountered when treating a larger gland. If pubic arch interference still occurs, the lithotomy can be slightly exaggerated to better open the perineum for access.
After the perineum is shaved, prepped, and draped, we use an Ioban dressing to elevate the scrotum off the perineum. A Foley catheter is sterilely placed into the bladder and clamped, allowing the bladder to be filled with urine or fluid to provide an acoustic window against which to better view the prostate. The transrectal ultrasound probe is placed per rectum. The cryotherapy grid is placed against the perineal surface, and the patient’s anatomy is viewed [see section “Using transrectal ultrasound to define pelvic anatomy prior to treatment” above for more detail].
Developing an appreciation of the ultrasonic appearance of a patient’s anatomy should not be understated. One needs to definitively know the location of the urinary sphincter, the rectal wall, and where the seminal vesicles/ducts insert into the prostate. Often, one has to rotate the stage of the ultrasound transducer away from the midline to better appreciate these structures. The relationship of the seminal vesicles to the prostate is interesting and almost unique to each patient. Some seminal vesicles insert very close to the base, while others can be seen posterior to the prostate extending to the apex or mid-region. Appreciating the sonographic characteristics of the seminal vesicles compared to the prostate is important before commencing the application of cryotherapy.
It is sometimes challenging to appreciate the location of the urinary sphincter when viewing sagittally along the midline prostate. A better means to view the location/junction of the urinary sphincter to the prostatic apex is to begin viewing laterally in the sagittal plane where the levator and sphincteric musculature is more readily appreciated. Once one finds the levators laterally, one can then rotate the probe medially in the sagittal plane to determine where these muscles become confluent with the urinary sphincter. It is important to note whether or not a patient has a median lobe. Many cryotherapists do not treat the median lobe as it would pose potential risk of injury to the ureteral orifices in select cases. In addition, the majority of median lobes are not malignant.
Measurements of height and width of the prostate (Fig. 11.11a) are then taken at the widest transaxial dimension followed by prostate length (base to apex) in the sagittal direction (Fig. 11.11b). The prostate volume is calculated, and the surgeon then decides on the number and types of cryoprobes to utilize. For example, one particular technology allows the surgeon to preset the length of lethal ice, while other technologies will grow a fixed length of ice. If the surgeon is planning to use variable ice length settings, we typically advise taking three measurements along the sagittal axis of the prostate: one measurement at the anterior region, one in the midsagittal plane, and one in the posterior region. The cryoprobes that are then subsequently placed into these locations can have the precise ice measurements set for their lethal kill zones.
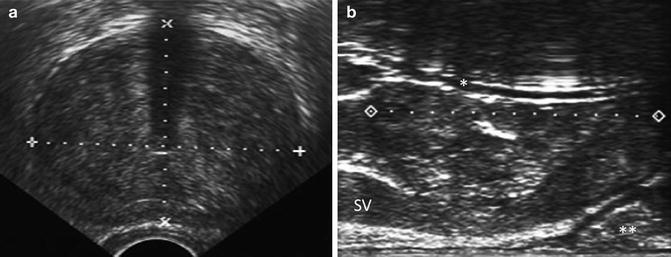

Fig. 11.11
(a) Transverse view of the mid-prostate. Volumetric measurements are being taken in both the anterior-posterior dimension (“height”) and the width. Note that the urethra is near the exact middle of the prostate in this case, and the acoustic shadowing cast by the indwelling catheter is seen in the far field. (b) Sagittal view of the prostate with a Foley catheter (*) running through the urethra. The sagittal length is measured from apex to base. Note that the prostate is measured at the widest part in the sagittal view, which typically is the midline segment. Note that in this particular patient, the urethra runs anterior to the widest length of the prostate. The seminal vesicle (SV) is identified, layered between the hyperechoic fat of Denonvilliers fascia and the posterior wall of the prostate. The posterior capsule of the prostate gently sweeps anteriorly toward the apex. In the uncompressed rectal wall, a wider section of the rectum (**) is usually identified toward the prostatic apex. In layman’s terms, this is often referred to as the “rectal wall hump”
In general, the manufacturer provides recommendations regarding a particular product’s ability to deliver spherical ice of various lengths and diameters attaining approximate temperatures in the 0 °C, −20 °C, and −40 °C zones [isotherms]. However, the operator needs to be sure that when these cryotherapy probes are placed, there is sufficient overlap of the ice balls, obviating any “warm pockets” or “variable freeze zones” that suggest untreated, potentially viable parenchyma (Fig. 11.8b). Alternatively, computer-generated cryoprobe placement schemes are commonly available on the manufacturer’s cryotherapy generators. Computerized models can be a means to help guide probe placement, but ultimately this responsibility rests with the cryosurgeon. Finally, the operator has the ability to measure distances between the probe and the urethra, the probe and the capsule, and between probes. One should consult the manufacturer’s recommendations for guidelines regarding these measurements.
We typically place the two anterior probes first. Each probe is localized and then placed using the transverse ultrasound view. The depth of the probes into the parenchyma is determined in sagittal view with each needle tip positioned at the base of the prostate. It is important to realize that there is little lethal ice generated beyond the tip of the cryoprobe, and for this reason the probes are placed right to the prostatic base/bladder wall junction. Typically, two probes are placed in the second or mid-transverse row and anywhere from two to four probes are placed in the posterior row. Again, the depths of all probes should be confirmed using the sagittal ultrasound view. It is important to also note that when viewing probe placement from a sagittal dimension, each individual probe should be placed horizontally (Fig. 11.12a). If there is any torque placed on the cryoprobe during placement, one may end up with skewed, or a non-horizontal probe placement, increasing the likelihood of treatment failure (Fig. 11.9a, b).
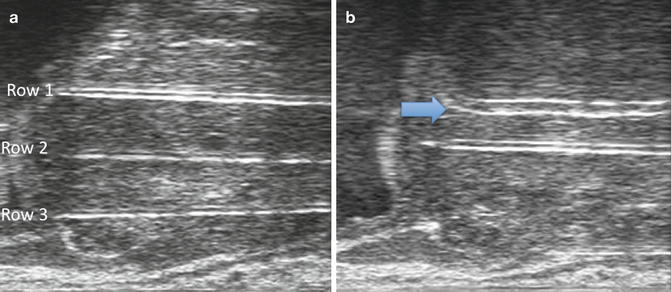

Fig. 11.12
(a) Sagittal view of the prostate with three rows of cryotherapy probes that have been positioned. It is common for these three rows of probes to be in different planes and not be seen simultaneously as is viewed in this image. (b) Sagittal view of the prostate. Note that row 1 (the anterior row) has been activated, and the hyperechoic rim of ice (arrow) can be seen slightly posterior to the cryoprobe. When the probe is first activated, one does not see much hypoechoic shadowing beyond the probe as the ice front has not had sufficient time to become dense. The second row probe (still not activated) can be seen posterior to the first row
The cryoablation kits include a number of thermocouples that can be utilized at the discretion of the treating surgeon. Many cryotherapists will place one thermocouple at the urinary sphincter and another at Denonvilliers fascia to monitor temperature in these critical regions. Other areas that surgeons may place thermocouples include the neurovascular bundle or areas of known cancer where one wants to make sure the temperature achieved is sufficiently lethal.
Following placement of the probes, the Foley catheter is removed and cystoscopy is performed. It is important to ensure that there is no violation of the urethral mucosa or bladder wall with a cryoprobe. If this is recognized, there is the opportunity to reposition the probe(s) and still proceed with the intended procedure. A super stiff guidewire is then passed as the cystoscope is withdrawn. A lubricated urethral warming catheter is then placed and secured to the drape. We recommend performing a final ultrasound verification of probe placement before beginning cryoablation. It is often helpful to visually inspect the insulated hubs of the needles externally to ensure that they are all aligned within a few millimeters of each other.
Two freeze-thaw cycles then ensue. Freezing is begun anteriorly. It is important to verify that the entire intended length of the cryo-active probe generates ice, and this can be visualized under sagittal ultrasound guidance (Fig. 11.12b). Following the first few minutes of freezing of the anterior rows, freezing of the second row typically commences. The entire freezing process should be monitored under the sagittal axis of ultrasonography. On the sagittal view, the entire length of ice may be monitored as compared to only viewing a “slice” of the field in the transverse plane. Many cryotherapists initially run the bottom or most posterior row of cryoprobes on a slower setting. For example, the posterior row can be run on approximately 60 % to allow the tissues in the posterior zone to become sufficiently cold before the ice front reaches the rectal wall. Most devices allow the operator to set the rate of cryotherapy between 10 and 100 % in increments of 10 %.
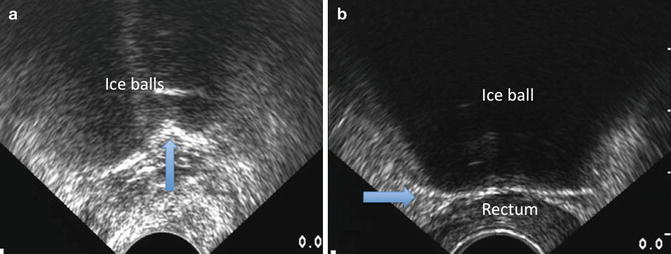
Cryotherapy can be monitored by both temperature thermocouples and ultrasonographic visualization of the ice. As the ice front advances, one sees the hyperechoic edge of the ice ball and an anechoic area of thick ice beyond it as the sound waves are unable to penetrate the dense ice structure (Fig. 11.13a, b). It is important when viewing the completion of the posterior row freeze to ensure that the ice is coming down evenly and that one side does not get ahead of the other (Fig. 11.13a). Denonvilliers fascia should be widened to allow sufficient space to run the lethal isotherm of the ice ball beyond the prostatic capsule. This can be achieved by decreasing the pressure placed by the transrectal ultrasound probe off the prostate by dropping the stage of the stepper posteriorly. Alternatively, saline can be injected into Denonvilliers space to widen the area. Cryotherapy is judged to be complete based upon the sagittal view of the ultrasound demonstrating the ice edge at the rectal wall (Fig. 11.10b). Ideally, temperatures of at least minus 20 °C should be attained at the posterior capsule of the prostate.
 < div class='tao-gold-member'>
< div class='tao-gold-member'>





Only gold members can continue reading. Log In or Register to continue
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree








