Chapter 39 Intrahepatic stones
Overview
Although hepatolithiasis is a rare disease prevalent only in East Asia—including China, Hong Kong, Korea, and Japan—it has increasingly been encountered in Western countries (Herman et al, 2005; Vetrone et al, 2006; Catena et al, 2006; Nuzzo et al, 2008; Al-Sukhni et al, 2008). Patients with intrahepatic stones follow a specific clinical course characterized by recurrent cholangitis with recurrent stones or liver abscess, which may cause pyogenic sepsis or liver dysfunction and result in biliary cirrhosis. Advances in hepatobiliary diagnostic modalities have allowed more precise delineation of the biliary anatomy and extent of disease, and the application of modern treatment modalities has resulted in a longer stone-free period over the past 2 decades. Intrahepatic stones include brown pigment stones (calcium bilirubinate stones), cholesterol stones, and other rare types. The majority of intrahepatic stones are brown pigment stones. Some centers, not only in Asia but also in Western countries, have reported increasing numbers of patients with primary cholesterol stones originating in the liver.
Definition and Surgical Anatomy
Intrahepatic stones are defined as concretions existing in the intrahepatic bile ducts. Although the confluence of the hepatic ducts is situated outside of the parenchyma of the liver, for convenience an intrahepatic bile duct is defined as any bile duct proximal to the confluence of the right and left hepatic ducts. Established by the Japanese Ministry of Health, Labor, and Welfare, the Japan Research Group for the Study of Hepatolithiasis proposed a classification of patients with hepatolithiasis into two groups: type I patients have stones only in the intrahepatic bile duct, and type IE patients have stones in the intrahepatic and extrahepatic bile ducts. Patients are classified further by location of stones within the liver: right side (type R), left side (type L), right and left sides (type LR), and caudate lobe (type C) (Nimura et al, 2005).
In Japan, the proportion of type I intrahepatic stones was 20.6% from 1975 through 1979, increasing to 45.5% from 1989 through 1992 (Tanimura et al, 1994). In Hong Kong, type I constituted 51% of intrahepatic stones from 1991 to 1996 (Liu et al, 1998). In Kaohsiung, Taiwan, the percentage of type I stones was 18.3% from 1980 through 1986 (Yuan et al, 1990). In addition to the findings in East Asian countries, type I intrahepatic stones were diagnosed in 77.3% of patients between 1986 and 2003 in Bologna, Italy (Vetrone et al, 2006).
Many patients with intrahepatic stones are reported to have no stones in the gallbladder. Yuan and colleagues (1990) reported that only 62 (30.7%) of 202 patients with intrahepatic stones had stones in the gallbladder. Regarding distribution of stones in the liver, type L is reported to be dominant. In Japan, the rate was 45.5% for type L, 26.1% for type R, and 26.3% for type LR for 1989 through 1992. The distribution was 52.1% (type L), 11.5% (type R), and 31.3% (type LR) in Hong Kong and 55% (type L), 18.3% (type R), and 26.7% (type LR) for 1989 through 1992 and 71.5% (type L), 16.2% (type R), and 12.2% (type LR) from 2000 through 2005 in Taiwan (Lee et al, 2007). Similarly, it was 59.0% (type L), 18.3% (type R), and 22.7% (type LR) for 1986 through 2003 in Italy (Vetrone et al, 2006). The incidence of stones in the caudate lobe (type C) was reported to be 6.6% (Nimura et al, 1998).
Hepatobiliary radiologists, endoscopists, and surgeons should have a thorough knowledge of the segmental and subsegmental anatomy of the intrahepatic bile ducts for preoperative diagnosis of intrahepatic stones, for an understanding of the variations of the intrahepatic biliary tree, and for decision making about therapeutic approaches to difficult biliary stenosis (see Chapter 1A, Chapter 1B ). The subsegmental anatomy of the intrahepatic bile ducts (Fig. 39.1) was established on the basis of clinical cholangiograms (Nimura, 1997). This cholangiographic anatomy is related to the radiologic study of the intrahepatic portal branches (Takayasu et al, 1985). The left lateral segmental ducts (B2, lateral posterior branch; B3, lateral anterior branch) join just behind the umbilical portion of the left portal vein. The left medial segmental duct (B4) enters this common trunk on the right of the umbilical portion of the left portal vein and forms the left hepatic duct. Segmental ducts B3 and B4 may make a common trunk that B2 joins to form the left hepatic duct. The right anterior segmental branches are superimposed over the right posterior segmental branches and are difficult to distinguish from each other on clinical cholangiograms taken with the patient in a supine position; they are divided clearly by the right hepatic vein on cholangiograms taken with the patient in the right lateral position, the right anterior in the left cranial area, and the right posterior in the right caudal area. The right posterior segmental duct joins the left hepatic duct in 12.6% of Taiwanese patients and 16.8% of Japanese patients with intrahepatic stones. This is an important anatomic variation when considering the distribution of intrahepatic stones (Kitagawa et al, 2003).
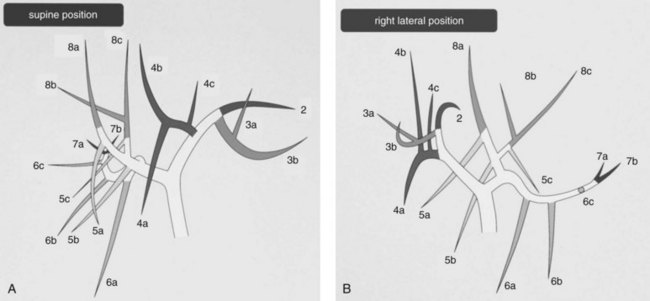
FIGURE 39.1 Cholangiographic anatomy of the intrahepatic segmental ducts according to the patient’s position. Numerals refer to Couinaud’s segments. 3a, Superior branch; 3b, inferior branch; 4a, inferior branch; 4b, superior branch; 4c, dorsal branch; 5a, ventral branch; 5b, dorsal branch; 5c, lateral branch; 6a, ventral branch; 6b, dorsal branch; 6c, lateral branch; 7a, ventral branch; 7b, dorsal branch; 8a, ventral branch; 8b, lateral branch; 8c, dorsal branch (Nimura et al, 1997).
Pathology
Intrahepatic calcium bilirubinate stones are composed mainly of bilirubin, cholesterol, fatty acids, and calcium. They are dark brown, soft, and friable. On a cut surface, a lamellar structure can be seen (Fig. 39.2). Stones are frequently located in the large bile ducts, such as the common, right, or left hepatic ducts. Diffuse dilation of the biliary tree, chiefly of the stone-containing ducts and their merging peripheral branches, and stenosis-like ductal lesions distal to the stones are commonly noted.
The histologic changes of the bile ducts with calcium bilirubinate stones are classified as either 1) chronic proliferative cholangitis, 2) suppurative cholangitis, or 3) chronic granulomatous cholangitis (Nakanuma et al, 1981). Chronic proliferative cholangitis consists of extensive proliferation of fibrous connective tissue in the ductal wall, moderate to severe infiltration of the inflammatory cells (primarily lymphocytes), and proliferation of mucin-producing glandular elements in the ductal wall. Suppurative cholangitis is characterized by ulceration and suppurative inflammation of the ductal wall. Chronic granulomatous cholangitis is characterized histologically by prominent granulomatous changes in the ductal wall and periductal tissues. In chronic proliferative cholangitis, histologic observation shows that most proliferative glands in the ducts have mucin-producing activity. These glands are observed to secrete a large amount of sialylated and sulfated acid mucins into the lumen (Maki et al, 1971; Sasaki et al, 1996). The affected part of the liver frequently shows atrophic changes. When the whole liver is involved, patients with intrahepatic stones may develop biliary cirrhosis and portal hypertension.
Intrahepatic cholesterol stones are composed mainly of cholesterol. They are yellow, small, and hard (Fig. 39.3). On a cut surface, a radial or crystalloid structure can been seen. Bile ducts containing cholesterol stones generally show a milder degree of fibrosis and glandular hyperplasia than ducts with calcium bilirubinate stones. Foamy cell aggregates and multinucleated giant cells are characteristic findings in patients with intrahepatic cholesterol stones.
Long-standing chronic cholangitis with biliary epithelial regeneration may be causally related to its malignant transformation, and several studies have demonstrated cholangiocarcinogenesis related to hepatolithiasis. Intraductal papillary growth of neoplastic epithelia in the liver (IPNL) is associated with hepatolithiasis and often displays variable gastroenteric metaplasia and significant intraductal spread. A continuous histologic spectrum may be observed, from papillary growths with low-grade and high-grade dysplasia to carcinoma in situ and finally to invasive carcinoma, particularly mucinous carcinoma (Chen et al, 2001). Mucin-secreting tumors of the intrahepatic biliary tract show frequent expression of MUC2 and nuclear CDX2. MUC2 expression in intestinal differentiation is closely related to the expression of CDX2 in IPNL, and mucinous intrahepatic cholangiocarcinoma (ICC) is associated with hepatolithiasis in the same manner as intestinal metaplasia in gastric mucosa and Barrett esophagus. This suggests that a common pathway of carcinogenesis may be involved in ICC with hepatolithiasis, gastric carcinoma, and adenocarcinoma in Barrett esophagus, in which CDX2-dependent intestinal metaplasia develops (Ishikawa et al, 2004). Further studies revealed that two types of neoplastic lesions preceding invasive ICC were identified: a flat-type neoplastic lesion called biliary intraepithelial neoplasia (BilIN) and intraductal papillary neoplasm of the bile duct (IPNB) or IPNL. Multistep carcinogenesis has been suggested in both lesions, and immunophenotypes of MUCs and cytokeratins might characterize three cholangiocarcinogenetic pathways in hepatolithiasis. IPNB and increased expression of MUC1 in BilIN are associated with tubular adenocarcinoma, and colloid carcinoma in IPNB is characterized by MUC1-negativity and less advanced pathologic stages (Zen et al, 2006).
Further immunohistochemical studies reveal that decreased membranous expression of β-catenin and E-cadherin is an early event in the tumorigenesis of both BilIN and IPNB lineages. However, different expression of cancer-related molecules was suggested in the progression of the BilIN and IPNB lineages in cholangiocarcinogenesis related to hepatolithiasis. The expression of matrix metalloproteinase (MMP) 7 and membrane type (MT) 1 MMT was closely associated with invasive growth of the BilIN lineage, and the Wnt signaling pathway may play an important role in the tumorigenesis of the IPNB lineage (Itatsu et al, 2007). The reported incidence of ICC detected at operation varies from 1.4% to 13% in Asian countries (Jeng et al, 1996; Liu et al, 1998), and it was 8.6% (three of 35 cases) in an Italian hospital (Nuzzo et al, 2008). A report from Hong Kong that assessed the long-term efficacy of hepatic resection for stone clearance in 103 patients noted cholangiocarcinoma in 10 patients (four known preoperatively) at the time of operation and in another three patients during follow-up (Chen et al, 2004). A similar report from Taiwan showed that three (2.4%) of 123 patients with intrahepatic stones had associated cholangiocarcinoma at the time of hepatectomy, and two (1.6%) had cholangiocarcinoma develop during a median follow-up of 40 months (range, 5 to 58 months) (Lee et al, 2007). Patients with retained stones or recurrent hepatolithiasis after percutaneous transhepatic cholangioscopic lithotomy have a high incidence of cholangiocarcinoma; four (17%) of 23 cases during a median follow-up of 11 years (range, 1 to 23 years; Chen et al, 2005). Intrahepatic stones associated with ICC are calcium bilirubinate stones in 89.1% of reported cases (57 of 64 cases; Sato et al, 1998). An autopsy case of intrahepatic cholesterol stones with peripheral ICC was reported, in which the causal relationship between the stones and the carcinoma remained speculative (Terada et al, 1989).
Etiology
Most intrahepatic stones appear as brown pigment (calcium bilirubinate) stones on macroscopic inspection and infrared analysis. The chemical composition of intrahepatic calcium bilirubinate stones is not identical to that of brown pigment stones in the extrahepatic bile ducts; the former contain more cholesterol and less bilirubin and bile acid and include lesser amounts of bile acids modified by bacterial metabolism (Shoda et al, 1991, 2003; Yamashita et al, 1988). The high incidence of bactibilia in patients with intrahepatic calcium bilirubinate stones suggests a relationship between bacteria and formation of stones (Maki, 1966). Among the bacteria in bile, Escherichia coli, Clostridium spp., and Bacteroides spp. show β-glucuronidase activity and are thought to be responsible for the hydrolysis of bilirubin glucuronide, the water-soluble form of bilirubin in bile, to free unconjugated bilirubin, which is water insoluble and combines with ionized calcium in bile to form calcium bilirubinate; this leads to stone formation. Bacterial infection alone cannot explain why intrahepatic brown pigment stones contain a higher amount of cholesterol than stones in the extrahepatic ducts. Upregulated cholesterogenesis and downregulated bile acid synthesis in the liver may be one of the causative factors responsible for the formation of hepatic bile substantially supersaturated with cholesterol, which leads to subsequent formation of cholesterol-rich stones (Shoda et al, 1995, 2003).
It is suggested that the risk factors of intrahepatic stone formation after surgery for choledochal cyst are anastomotic stricture, residual debris in the intrahepatic ducts, and dilated intrahepatic bile ducts. A Japanese surgical study reported secondary hepatolithiasis that developed after choledochal cyst excision with hepaticoenterostomy (CEHE). Although no stone formation was seen in the 145 children who had CEHE at the age of 5 years or younger, three (5.5%) of 55 children older than 5 years who underwent CEHE had intrahepatic stones develop. On the other hand, intrahepatic stones developed in five (12.5%) of the 40 adult patients with CEHE. Two of the five developed anastomotic stricture of the end-to-side hepaticojejunostomy distally to the stone at the porta hepatis with or without left intrahepatic duct dilation. The incidence of post-CEHE hepatolithiasis in children aged 5 years or younger was significantly lower than in other children and adults. In addition to the hepatolithiasis, one adult developed ICC at age 54 years, 6 years after CEHE converted from cholecystectomy performed 16 years previously (Yamataka et al, 1997). On the other hand, intrahepatic stones developed in patients with biliary atresia who had undergone hepatic portoenterostomy, and almost 100% calcium bilirubinate stones were found in bile lake in the resected liver (Tainaka et al, 2006).
A study from Taiwan investigated the possible association between biliary helminthic infestation (ascariasis or clonorchiasis) and hepatolithiasis (Huang et al, 2005). By using immunologic techniques, the authors showed a higher prevalence of ascariasis and clonorchiasis in patients with intrahepatic stones than in control subjects (33.6% vs. 17.4%; P = .005). However, analysis of patients with hepatolithiasis with and without evidence of helminthic infestation showed no differences in the rates of biliary stricture formation, stone recurrence, secondary biliary cirrhosis, or cholangiocarcinoma. An epidemiologic study suggested that lower socioeconomic and hygienic status may be involved in stone formation in Taiwan (Momiyama et al, 2008). In the Kami-Goto Islands of Japan, where incidence of this disease is high, there were similar hygienic and social problems from the 1960s to the 1980s. However, there has been no new case of hepatolithiasis in the generation born after the 1970s, after improvements in the environment and economic status (Yasaka, 2008).
The most important factors for the formation of intrahepatic calcium bilirubinate stones are biliary stasis, bacterial infection, and biliary mucin produced from the covering and proliferated mucous gland epithelium of the intrahepatic bile ducts. Calcium bilirubinate and fatty acid–calcium soap occur through hydrolysis of bilirubin conjugates and lecithin by bacterial β-glucuronidase and phospholipase in bile. These salts can precipitate and form microcalculi, particularly if a nidus is present, such as desquamated epithelium or biliary mucin (Forstner & Forstner, 1975). These microcalculi coalesce to form large stones. Chronic proliferative inflammation seems to precede bile duct damage: the development of intrahepatic stones leads to biliary strictures, which promote further propagation of stones through alterations of bile flow (Nakanuma et al, 1988).
A study on the biliary dynamics of patients with intrahepatic stones revealed that the time-activity curves of technetium-99m-ethylenediaminetetraacetic acid (EDTA) were prolonged significantly in affected and apparently unaffected intrahepatic bile ducts compared with the curves in normal subjects (Takahashi et al, 1986). This result suggests a global impairment of bile flow, even when only one side of the liver is affected by stones. The curves in the intrahepatic bile ducts of the left lobe were more prolonged than those of the right lobe, even in normal liver; this prolongation could explain why involvement of the left lobe is more common.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree









