CHAPTER 114 Intestinal Ischemia
Since the development and widespread use of colonoscopy, angiography, computed tomography (CT), and other imaging modalities, various types of ischemic injury to the gastrointestinal tract have been recognized and increasingly appreciated (Table 114-1; see also Tables 114-2 and 114-4). Our concepts of their pathogenesis, diagnosis, and management have been so altered since the 1980s that much of what has been written in the past is no longer applicable. In this chapter we describe the spectrum of ischemic damage to the gastrointestinal tract and discuss the management of these conditions in light of recent advances.
Table 114-1 Types and Approximate Frequencies of Intestinal Ischemia
| TYPE | FREQUENCY (%) |
|---|---|
| Colon ischemia* | 75 |
| Acute mesenteric ischemia* | 25 |
| Focal segmental ischemia* | <5 |
| Chronic mesenteric ischemia | <5 |
* Includes mesenteric venous thrombosis. Mesenteric venous thrombosis can manifest as colon ischemia, acute mesenteric ischemia, or focal segmental ischemia.
ANATOMY OF THE SPLANCHNIC CIRCULATION
The celiac axis (CA), superior mesenteric artery (SMA), and inferior mesenteric artery (IMA) supply almost all of the blood flow to the digestive tract.1 There is marked variability of vascular anatomy among individuals, but typical patterns have emerged from anatomic dissections and abdominal angiographic studies.
CELIAC AXIS
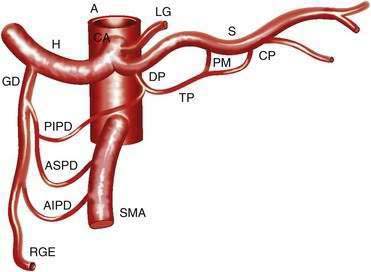
The CA (Fig. 114-1) arises from the anterior aorta and typically gives rise to three major branches: the left gastric artery, the common hepatic artery, and the splenic artery. The common hepatic artery gives rise to the gastroduodenal, right gastroepiploic, and anterior superior pancreaticoduodenal arterial branches. The splenic artery gives off pancreatic and left gastroepiploic arterial branches. The CA and its branches supply the stomach, duodenum, pancreas, and liver.
SUPERIOR MESENTERIC ARTERY
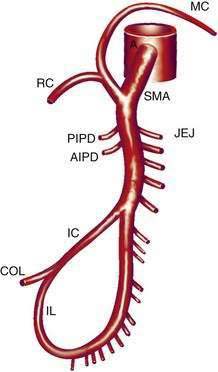
The SMA (Fig. 114-2) has its origin from the anterior aorta near the neck of the pancreas. It gives rise to five major vessels: the anterior and posterior inferior pancreaticoduodenal vessels, middle colic, right colic, and ileocolic arteries, as well as to a series of jejunal and ileal branches, all of which supply their named portions of intestine. These intestinal branches typically form a series of arcades, and from the terminal arcade, numerous straight vessels arise that enter the intestinal wall.
INFERIOR MESENTERIC ARTERY
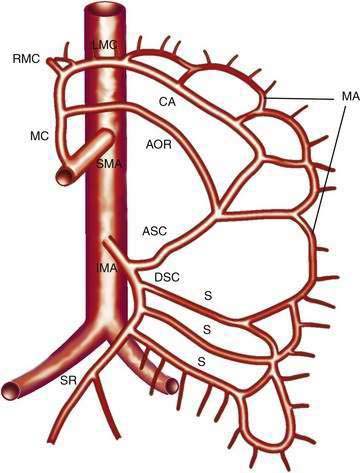
The IMA (Fig. 114-3) arises 3 to 4 cm above the aortic bifurcation close to the inferior border of the duodenum. It branches into the left colic artery, gives off multiple sigmoid branches, and terminates as the superior rectal artery. The IMA and its branches supply the large intestine from the distal transverse colon to the proximal rectum. The distal rectum is supplied by branches of the internal iliac (hypogastric) artery.
COLLATERAL AND ANASTOMOTIC CIRCULATION
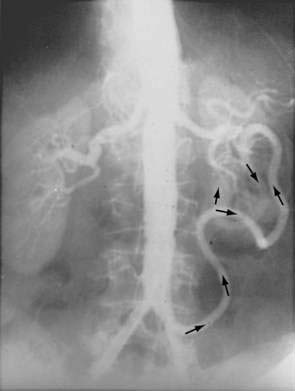
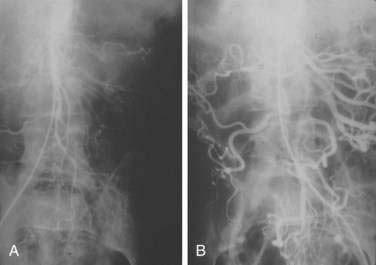
There are three potential paths of communication between the SMA and IMA: the marginal artery of Drummond, which is closest to and parallel with the wall of the intestine; the central anastomotic artery, a larger and more centrally placed vessel; and the arc of Riolan, an artery in the base of the mesentery. In the presence of SMA or IMA occlusion, a large collateral termed the meandering artery may be identified angiographically and represents a dilated central anastomotic artery or arc of Riolan (Fig. 114-4). It is critical to determine the direction of flow within a meandering artery before sacrificing the IMA, such as during aortic aneurysm surgery, lest the IMA be the main vessel supplying blood to the small bowel because of an occluded SMA.
PATHOPHYSIOLOGY AND PATHOLOGY
Ischemic injury of the intestine results from deprivation of oxygen and nutrients necessary for cellular integrity. Remarkably, the bowel can tolerate a 75% reduction of mesenteric blood flow and oxygen consumption for 12 hours with no changes on light microscopy, because only one fifth of the mesenteric capillaries is open at any time, and when oxygen delivery is decreased, the bowel adapts by increasing oxygen extraction.2 Below a critical level of blood flow, however, these compensatory mechanisms are overwhelmed and no longer protective.
Ischemic damage results both from hypoxia during the period of ischemia and reperfusion injury when blood flow is re-established. More reinjury from brief ischemia appears during reperfusion, but as the ischemic period lengthens, hypoxia becomes more detrimental than reperfusion3; the injury after three hours of ischemia and one hour of reperfusion is more severe than that after four hours of ischemia. Reperfusion injury has been attributed to many factors, including reactive oxygen radicals. When molecular oxygen is reduced in univalent steps, superoxide, hydrogen peroxide, and hydroxyl radicals are formed. These oxygen radicals damage an array of molecules found in tissues, including nucleic acids, membrane lipids, enzymes, and receptors; such widespread damage can result in cell lysis, impaired cell function, and necrosis on reperfusion of ischemic tissues.
Neutrophils are another source of reactive oxygen metabolites. During reperfusion, XO-derived oxidants initiate the production and release of leukotriene B4 and platelet-activating factor, which lead to neutrophil adherence and migration. The adherent leukocytes mediate microvascular injury by release of proteases and physical disruption of the endothelial barrier. Oxygen radical scavengers (superoxide dismutase, dimethyl sulfoxide), XO inhibitors, and agents that inhibit leukocyte adherence and migration have been shown experimentally to protect various organs against reperfusion injury, but are not yet used clinically because, in large measure, they must be given before or coincident with the ischemic injury to have protective effects.3
ACUTE MESENTERIC ISCHEMIA
Intestinal ischemia can be classified as acute or chronic and of venous or arterial origin. In the acute forms, intestinal viability is threatened, whereas in the chronic forms, blood flow is inadequate to support the functional demands of the intestine. Acute mesenteric ischemia (AMI) is much more common than the chronic type, and arterial disease is more common than venous disease. Arterial forms of AMI include SMA embolus (SMAE), nonocclusive mesenteric ischemia (NOMI), SMA thrombosis (SMAT), and focal segmental ischemia (FSI) (Table 114-2). Acute mesenteric venous thrombosis (MVT) and FSI are the venous forms of AMI.
Table 114-2 Causes and Approximate Frequencies of Acute Mesenteric Ischemia
| CAUSE | FREQUENCY (%) |
|---|---|
| SMA embolus | 50 |
| Nonocclusive mesenteric ischemia | 25 |
| SMA thrombosis | 10 |
| Mesenteric venous thrombosis | 10 |
| Focal segmental ischemia | 5 |
SMA, superior mesenteric artery.
INCIDENCE
Most series of AMI reported in the late 1970s and early 1980s showed that SMAE was responsible for 40% to 50%, NOMI for 20% to 30%, and SMAT for 10% to 20% of cases. The incidence of NOMI has now declined, however, likely because intensive care unit monitoring enables prompt correction of hypotension and blood volume deficits, and the widespread use of calcium channel blockers and other systemic vasodilators might protect the vascular bed from spasm. Today, SMAE is the most common cause of AMI. In one study of autopsies on patients who died from acute mesenteric thromboembolic occlusion the embolus-to-thrombus ratio was 1.4 : 1.4
CLINICAL FEATURES
Unexplained abdominal distention or gastrointestinal bleeding may be the only indications of AMI when pain is absent, especially when AMI is due to NOMI. Distention, although absent early in the course of AMI, is often the first sign of intestinal infarction. The stool contains occult blood in 75% of patients. Right-sided abdominal pain associated with the passage of maroon or bright red blood in the stool, although characteristic of colon ischemia, also may be seen with AMI, because the blood supply to the right colon and small intestine originates from the SMA. Elderly patients with AMI have been reported to develop mental confusion acutely in as many as 30% of cases.5 In patients who survive cardiopulmonary resuscitation and who then develop recurrent bacteremia or sepsis, the suspected cause of sepsis should be NOMI that resulted in a segment of bowel with subacute ischemic injury, acting as a portal for bacterial translocation.6 Although episodes of sepsis may be treated successfully with antibiotics, the length of damaged bowel must be removed to prevent recurrent sepsis.
LABORATORY FEATURES AND DIAGNOSIS
On admission to the hospital, approximately 75% of patients with AMI have leukocytosis greater than 15,000 cells/mm3 and about 50% have metabolic acidemia. A normal white blood cell (WBC) count cannot be used to exclude early AMI, just as a high WBC count does not make the diagnosis. Elevated levels of serum phosphate, d-lactate, amylase, and other enzymes have been noted, as have high peritoneal fluid amylase and intestinal alkaline phosphatase activity, but the sensitivity and specificity of these markers of intestinal ischemia have not been established.7 More-specific intestinal enzymes including diamine oxidase, hexosaminidase, glutathione S-transferase,8 and intestinal fatty acid-binding protein9 also lack sufficient sensitivity and specificity to diagnose AMI. Moreover, serum markers, when elevated, usually indicate late-stage disease.
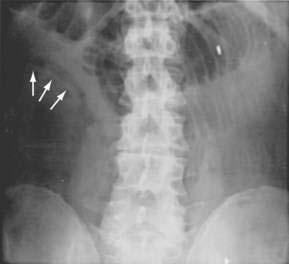
Although they are poorly sensitive (30%) and nonspecific, plain films of the abdomen still are obtained in evaluating patients with suspected AMI. Plain films of the abdomen usually are normal in AMI before infarction. Later on, formless loops of small intestine, ileus, thumbprinting of the small bowel or right colon (Fig. 114-5), or still later, pneumatosis and portal or mesenteric vascular gas may be seen. In one study, the mortality rate of patients with normal plain film studies was 29%, whereas it was 78% in those with abnormal findings.10 The primary purpose of plain films (or CT scans) is to exclude causes of abdominal pain other than ischemia that might mandate a different therapeutic approach.
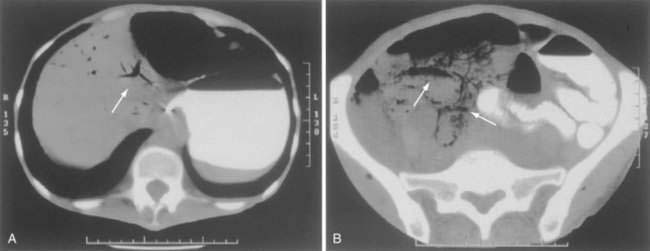
CT has largely replaced plain film study of the abdomen for diagnosis and is used to identify arterial and venous thromboses as well as ischemic bowel.11–14 Findings on CT include colon dilatation, bowel wall thickening, abnormal bowel wall enhancement, lack of enhancement of arterial vasculature with timed intravenous contrast injections, arterial occlusion, venous thrombosis, engorgement of mesenteric veins, intramural gas and mesenteric or portal venous gas (Fig. 114-6), infarction of other organs, ascites, and signs related to the cause of the infarcted bowel such as hernia.11 There are three relatively specific findings of AMI that are better depicted on CT scans compared with plain films: gas in the bowel wall or portal system, acute embolic infarction of other intra-abdominal organs, and thrombi in the mesenteric vessels.12 Unfortunately, the early signs on CT are nonspecific and the late signs reflect necrotic bowel.
In a study of 26 patients with AMI who had a preoperative multislice CT scan followed by exploratory laparotomy, CT scanning identified mesenteric arterial thrombosis in 16 of 17 patients and mesenteric vein thrombosis in 7 of 7 patients, all confirmed at operation. In this study, the sensitivity and specificity of CT scanning for occlusive AMI was 92% and 100%, respectively.15 The predictive value of CT scanning in the community might not be as high as in this report, because this study used only highly trained radiologists; improved CT scanner technology, however, likely will yield higher detection rates than in the past.
CT angiography has been shown to be promising in the diagnosis of AMI and, in one study, the added CT angiographic findings were believed to alter clinical management in 19% of 62 patients by making the diagnosis of AMI when CT alone did not.16 Magnetic resonance (MR) angiography and venography are newer imaging techniques used to diagnose AMI; they not only can image the vasculature but might be useful in determining metabolic consequences of inadequate blood flow.17,18
Selective mesenteric angiography, often with papaverine infusion, currently is the mainstay of diagnosis and initial treatment of both occlusive and nonocclusive forms of AMI, and it should be performed promptly if AMI is suspected or diagnosed on other imaging tests. Sensitivity and specificity of mesenteric angiography for diagnosing AMI in most studies are 90% to 100% and 100%, respectively.19 Opponents of routine angiography for patients with suspected AMI cite several problems with this approach:
TREATMENT
Our approach to the management of AMI is based on several observations. First, if the diagnosis is not made before intestinal infarction, the mortality rate is 70% to 90%. Second, diagnosis of both the occlusive and nonocclusive forms of AMI can be made in most patients by angiography. Third, vasoconstriction, which can persist even after the cause of the ischemia is corrected, is the basis of NOMI and a contributing factor in the other forms of AMI. Finally, vasoconstriction can be relieved by vasodilators infused into the SMA. The cornerstones of our approach, therefore, are the earlier and more liberal use of angiography and the incorporation of intra-arterial papaverine in the treatment of both occlusive AMI and NOMI. Duration of symptoms parallels mortality, and therefore early diagnosis and treatment is paramount to increase the chance for survival.20
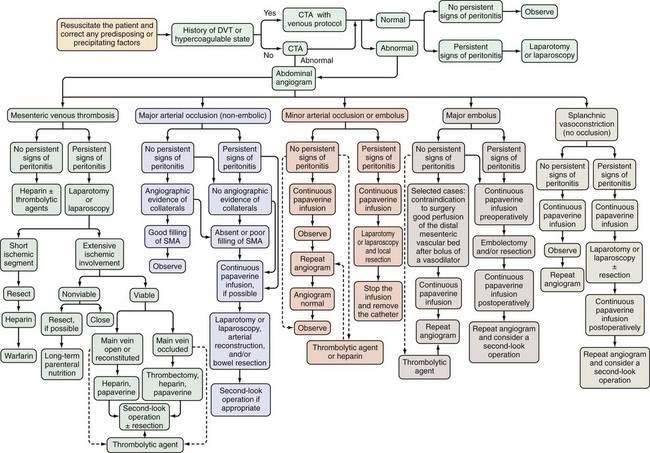
AMI should be suspected in patients older than 50 years who have the risk factors previously described and in younger patients—especially those with atrial fibrillation, vasculitis, a coagulation disorder, and those on vasoactive medications—who seek medical attention for sudden, severe abdominal pain that lasts longer than several hours. These patients should be managed according to the algorithm shown in Figure 114-7. Less-absolute indications for inclusion into this protocol consist of unexplained acute abdominal distention, colonoscopic evidence of isolated right-sided colonic ischemia, and acidosis without an identifiable cause.
Initial management of patients with suspected AMI includes resuscitation and diagnostic imaging studies. Resuscitation includes relief of acute congestive heart failure and correction of hypotension, hypovolemia, and cardiac arrhythmias. Broad-spectrum antibiotics (e.g., levofloxacin, metronidazole, piperacillin-tazobactam) are given immediately because of the high incidence of positive blood cultures in AMI and because they reduce the extent and severity of ischemic injury in experimental animals.21 There are no randomized, controlled trials showing the benefit of antibiotics in AMI, and it is unlikely that such trials will ever be done. After resuscitation, plain films or CT scan of the abdomen are obtained, not to establish the diagnosis of AMI but rather to exclude other causes of abdominal pain. A normal plain film or CT scan does not exclude AMI; ideally, patients are studied before radiologic signs appear because these signs connote irreversibly damaged bowel. If no alternative diagnosis is made on these studies, selective SMA angiography is performed. Based on the angiographic findings and the presence or absence of peritoneal signs, the patient is treated according to the algorithm in Figure 114-7.
Laparotomy is performed in AMI to restore arterial flow obstructed by embolus or thrombosis or to resect irreparably damaged bowel, or both. Embolectomy, thrombectomy, or arterial bypass precedes evaluation of intestinal viability because bowel that initially appears infarcted can show surprising recovery after adequate blood flow is restored. In the operating room, intestinal viability can be assessed clinically, by qualitative or quantitative surface fluorescence or by Doppler ultrasonography.22 Animal models show that administration of intravenous glucagon, intravenous heparin-binding epidermal growth factor (HB-EGF)-like growth factor or intraluminal nitroglycerin after revascularization of an acute arterial occlusion can improve mucosal viability and minimize reperfusion damage.23,24
Specific Types of Acute Mesenteric Ischemia
Superior Mesenteric Artery Embolus
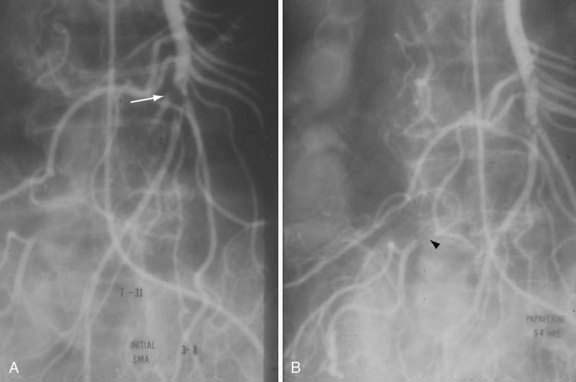
SMAE is responsible for 40% to 50% of AMI episodes. Emboli usually originate from a left atrial or ventricular mural thrombus. Many patients with SMAE have had previous peripheral artery emboli, and approximately 20% have synchronous emboli. SMAEs lodge at points of normal anatomic narrowing, usually immediately distal to the origin of a major branch. Angiography typically reveals a rounded filling defect with nearly complete obstruction to flow. Mesenteric atherosclerosis is usually not as severe as in SMAT. Emboli proximal to the origin of the ileocolic artery are considered major emboli. Minor emboli are those that lodge in the SMA distal to the takeoff of the ileocolic artery or in the distal branches of the SMA (Fig. 114-8).
Various therapeutic approaches have been proposed for SMAE, depending on the presence or absence of peritoneal signs, whether the embolus is partially or completely occluding, and whether the embolus is above the origin of the ileocolic artery or more distal. Therapy for SMAE has included surgical revascularization, intra-arterial perfusion with vasodilators or thrombolytic agents, and anticoagulation.19 In the absence of peritoneal signs, minor SMA emboli have been treated successfully with all of these agents without the need for surgery. Exploration is usually performed in patients with major emboli after papaverine infusion is begun. Nonoperative therapy using only papaverine infusion is attempted if there are significant contraindications to surgery, no peritoneal signs, and adequate perfusion of the vascular bed distal to the embolus after a bolus of vasodilator into the SMA.
Exploratory laparotomy is mandatory when peritonitis is present; embolectomy and bowel resection are performed as necessary. If possible, intra-arterial papaverine is begun before surgery and is continued during surgery. If no “second-look” operation is planned, infusion is continued for 12 to 24 hours postoperatively; persistent vasospasm is excluded by angiography before the catheter is removed (see Fig. 114-8). If a second operation is planned, the infusion is continued through the second procedure until angiography shows the vasoconstriction is ceased. Recognition of persistent vasoconstriction has prompted some authorities to recommend routine use of intra-arterial papaverine in all patients with SMAE; the best survival rates are seen in patients treated by this approach.19
Use of transcatheter thrombolytic therapy (e.g., alteplase or urokinase) can be considered before exploratory laparotomy if the patient does not have signs of peritonitis. Prospective studies and meta-analyses have shown that thrombolysis may be effective in resolving thrombi, improving symptoms, and avoiding surgery in patients with lesions amenable to such therapy.25,26 Thrombolytic therapy is most likely to be successful when the embolus is partially occluding or is minor and when the study is performed within 12 hours of the onset of symptoms.27 A canine study showed that intra-arterial streptokinase was more effective than intra-arterial papaverine in lysing clots implanted into the SMA, although greater ischemic damage occurred with streptokinase than with papaverine because of papaverine’s action to cause vasodilation and open collateral pathways for blood flow around the obstructing clot. When streptokinase and papaverine were administered simultaneously, neither medication functioned as well as it did alone and intestinal damage was intensified.28 Given the shortage of supporting evidence for thrombolytics in AMI and the high complication rate attending their use, this treatment remains controversial.27
Nonocclusive Mesenteric Ischemia
NOMI is responsible for 20% to 30% of AMI and usually is due to splanchnic vasoconstriction consequent to a preceding cardiovascular event. AMI can appear hours to days after the event, and vasoconstriction, which initially is reversible, can persist even after the precipitating event has been corrected. Precipitating causes for NOMI include acute myocardial infarction, congestive heart failure, arrhythmias, shock, cirrhosis, medications (e.g., digitalis), cardiopulmonary bypass surgery, and chronic kidney disease, especially when patients are on either hemodialysis or peritoneal dialysis. When presenting with abdominal pain, patients on peritoneal dialysis may be thought to have peritonitis, thereby delaying the diagnosis of NOMI and resulting in a poor outcome.29
NOMI is diagnosed by angiography using four criteria: narrowing of the origins of SMA branches, irregularities in the intestinal branches, spasm of the arcades, and impaired filling of intramural vessels. Patients with these signs who are neither in shock nor on vasopressors and who do not have pancreatitis can be considered to have NOMI (Fig. 114-9).
Acute Thrombosis of the Superior Mesenteric Artery
SMAT is demonstrated on flush aortography, which usually shows occlusion of the SMA 1 to 2 cm from its origin. Some distal filling of the SMA via collaterals is common. Branches proximal and distal to the obstruction can show localized or diffuse vasoconstriction. In patients with abdominal pain, no abdominal tenderness, and complete occlusion of the SMA on aortography, it is important, though difficult, to distinguish between acute thrombosis and long-standing, coincidental chronic occlusion. Prominent collaterals between the SMA and other major splanchnic vessels indicate chronic SMA occlusion. If there is good filling of the SMA, the occlusion is considered chronic and the abdominal pain is considered unrelated to mesenteric vascular disease (see Fig. 114-4). The absence of collateral vessels or the presence of collaterals with inadequate filling of the SMA indicates an acute occlusion and demands prompt intervention. If possible, an angiographic catheter is placed in the proximal SMA, and papaverine infusion is begun before surgery is undertaken.
Results
Although mortality rates of 70% to 90% were reported through the 1980s for patients whose AMI was diagnosed and treated conventionally, the approach described here can reduce these catastrophic figures. The best survival is reported in series in which angiography has been used routinely in the management of AMI.30–35
MESENTERIC VENOUS THROMBOSIS
INCIDENCE
In early studies, MVT was believed to be the major cause of AMI, but most of these cases probably represented NOMI. Today, only 5% to 10% of patients with AMI have MVT. The mean age at presentation with MVT is in the mid-60s.36 A Swedish study showed that the highest incidence of MVT was 11.3 per 100,000 person years among those 70 to 79 years old.37
PREDISPOSING CONDITIONS
Previously, a cause of MVT was identified in fewer than half of patients. The discoveries of the primary and secondary hypercoagulable states and the use of estrogens for contraception and hormone replacement have led to identification of the cause in almost 90% of patients.38 Arterial hypertension is the most commonly associated medical comorbidity with this disorder, and neoplasms (e.g., acute lymphocytic leukemia, adenocarcinoma of the pancreas, stomach) and coagulation disorders (e.g., lupus anticoagulant, factor V Leiden, and protein S deficiency) also are commonly seen.36,39 A list of predisposing conditions for MVT is given in Table 114-3.
Table 114-3 Conditions Associated with Mesenteric Venous Thrombosis