CHAPTER 110 Intestinal Infections by Parasitic Worms
Videos for this chapter can be found on www.expertconsult.com.
Parasitic worms are found worldwide. Modern travel, emigration,1 and consumption of “exotic” cuisines allow intestinal helminths to appear in any locale. People now acquire tropical helminths without leaving their industrialized temperate cities. Travel history is a critical, but often overlooked, aspect of the patient interview. Many helminths survive for decades within a host, so even a remote history of visits to or emigration from countries where they are endemic is important. Fresh food is flown around the world and often consumed raw.
In developed countries, we usually diagnose an intestinal helminth because we stumble across it rather than because we actively pursue it. Helminths are complex organisms well adapted to their hosts; like quiet house guests, most cause no symptoms. Worms rarely cause diarrhea, but many medical laboratories do not assay formed stool routinely for parasite eggs. Physicians need to communicate their concerns of possible helminthic infection to laboratory personnel. A telephone call to the local laboratory before a sample is sent can improve diagnostic results dramatically. Occasionally, alarmed patients bring proglottids or whole worms that they passed with their stools. These specimens should be fixed in 5% aqueous formalin and sent for identification.2 All specimens should be handled carefully with full precautions to avoid accidental exposure.
Some helminths can cause severe disease, but this is unusual. Most persons colonized with helminths have no symptoms or illness attributable to the parasites. Only with heavy infections does disease result. Well-adapted worms usually act more as commensals than as pathogens. It is even possible that exposure to helminths affords some protection against disease due to excessive immune reactions.3,4 Helminths induce immune regulatory pathways.5 Recent studies in mice and rats show that exposure to helminths can be used to prevent or treat colitis,3,6–8 insulin-dependent diabetes,9 and autoimmune encephalitis.10,11 Studies in humans show that helminth exposure improves ulcerative colitis12 and probably Crohn’s disease13,14 and that helminth eradication increases atopy.15 Although it remains important to treat helminthic infections when they are discovered, further research on these organisms can enable discovery of new approaches to treat immune-mediated disease.
NEMATODES
ASCARIS LUMBRICOIDES
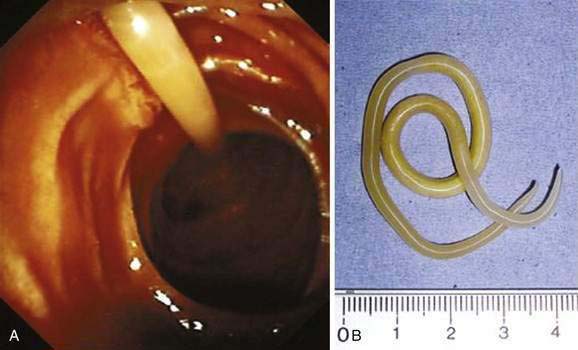
Ascaris lumbricoides is the largest of the nematode parasites that colonize humans. Females can grow to 49 cm (19 inches).16 The name “lumbricoides” alludes to its resemblance to earth worms (Lumbricus sp.). The parasite is acquired by ingesting its eggs. Ascaris can cause intestinal obstruction and pancreaticobiliary symptoms. Treatment is albendazole.
Epidemiology
A. lumbricoides has a worldwide distribution, although these parasites are most numerous in less-developed countries and in areas with poor sanitation. About 25% of the world’s population (1.2 billion people) harbor A. lumbricoides.17,18 Children acquire the parasite by playing in dirt contaminated with eggs, whereas adults most often are infected by farming or eating raw vegetables from plants fertilized with untreated sewage. Pigs harbor Ascaris suum, which is closely related to A. lumbricoides, but cross-infection is rare.19
Clinical Features and Pathophysiology
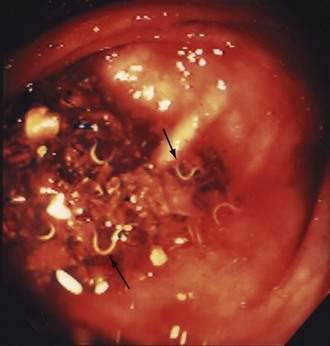
A. lumbricoides produces no symptoms in most infected persons. Often, worms are found unexpectedly on endoscopy20,21 (Video 110-1) or are seen on radiologic imaging,22 or eggs are identified in stool specimens of patients with symptoms not directly attributable to the worms. Disease usually develops only in those with heavy worm burdens: pulmonary, intestinal, and hepatobiliary ascariasis are well described.
Large numbers of mature worms can cause severe intestinal symptoms including abdominal pain, distention, nausea, and vomiting. The most common complication of intestinal ascariasis is partial or complete small bowel obstruction; such patients often have a history of passing mature worms in their stool or vomitus. Patients with intestinal obstruction generally have more than 60 worms,23 and the rare patients with fatal cases often have more than 600 worms. Fatality results from intestinal necrosis caused by obstruction, intussusception, or volvulus (Fig. 110-1).24 Most cases of obstruction, absent signs of peritonitis or perforation, can be managed conservatively.
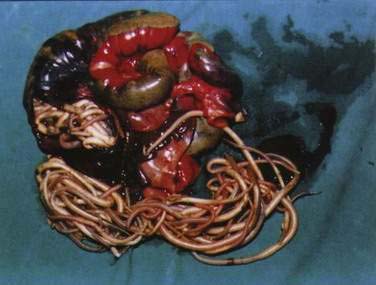
Figure 110-1. Small intestinal obstruction caused by Ascaris lumbricoides.
(From Wasadikar PP, Kulkarni AB. Intestinal obstruction due to ascariasis. Br J Surg 1997; 84:410.)
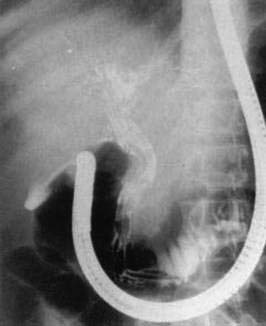
A. lumbricoides are highly motile. Mature worms can enter the ampulla of Vater (Fig. 110-2) and migrate into the bile or pancreatic ducts, causing biliary colic, obstructive jaundice, ascending cholangitis, acalculous cholecystitis, or acute pancreatitis.16 Pregnancy can promote biliary trespass.25 The worms can move in and out of the papilla, producing intermittent symptoms and fluctuating laboratory tests. Recurrent ascending cholangitis or acute pancreatitis from ascariasis is rare in highly developed Western countries but can be fatal if the diagnosis is not entertained.26
Diagnosis
Ascaris eggs are visible in direct smears of stool (Fig. 110-3). The eggs begin to appear in the stool about two months after initial exposure. Fertilized eggs are 35 by 55 µm and have a thick shell and outer layer; females also lay unfertilized eggs that are larger (90 by 44 µm) and have a thin shell and outer layer. Ascaris eggs that lose their outer layer resemble the eggs of hookworms.

Figure 110-3. Stool specimen containing helminth eggs. A, Ascaris lumbricoides. B, Hookworm. C, Trichuris trichiura. D, Fasciolopsis buski.
(A to D, Courtesy of Mae Melvin, MD, Atlanta, Ga.)
Adults worms may be seen at endoscopy,21 or identified on upper gastrointestinal series as long, linear, filling defects within the small intestine.22 The worms retain barium after it has cleared from the patient’s gastrointestinal tract, producing linear opacities. Similar findings are seen on endoscopic retrograde cholangiopancreatography (ERCP) if a worm is in the bile or pancreatic duct (Fig. 110-4). Ascaris also has a characteristic appearance on ultrasound examination of the biliary tree or pancreas: They appear as long, linear echogenic strips that do not cast acoustic shadows.22
Treatment
Asymptomatic colonization with A. lumbricoides is treated easily with a single 400-mg oral dose of albendazole. Albendazole inhibits glucose uptake and microtubule formation, effectively paralyzing the worms. Albendazole is poorly absorbed but is teratogenic, and therefore it should not be used in pregnant women. When possible, treatment with this agent should be delayed until after delivery. Single-dose mebendazole also is efficacious for Ascaris.27 A study of 1042 pregnant women in Peru found no adverse effect of a single 500-mg oral dose of mebendazole on birth outcomes.28
Hepatobiliary ascariasis also can be treated conservatively with fluid resuscitation, bowel rest, and antibiotics.29 Worms in the bile duct are not effectively treated with albendazole because it is poorly absorbed and not concentrated in the bile. This feature of albendazole is advantageous because were paralyzed worms unable to pass through the sphincter of Oddi, they could become trapped in the bile duct. Patients with hepatobiliary ascariasis should be treated with albendazole each day for several days because the worms only become susceptible when they migrate out of the bile duct.
Worms also can invade the pancreatic duct and can be treated conservatively, as for hepatobiliary ascariasis.30 Ascending cholangitis, acute obstructive jaundice, or acute pancreatitis requires emergent ERCP with worm extraction from the ducts by balloon, basket, or forceps—preferably without sphincterotomy. Ampullary sphincterotomy permits worms easier access to the ducts and can increase the risk of recurrent pancreaticobiliary ascariasis.31
STRONGYLOIDES STERCORALIS
Clinical Features and Pathophysiology
Most patients with S. stercoralis have no abdominal symptoms. Patients might have a serpiginous urticarial rash (larva currens) caused by the rapid (5 to 10 cm/hour) dermal migration of filariform larvae. This rash often occurs on the buttocks from larvae entering the perianal skin after they exit the anus during autoinfection. A study of prisoners of war found this creeping eruption to be a far more common symptom of chronic strongyloidiasis than were gastrointestinal complaints.32 Occasionally, patients have nausea, abdominal pain, or unexplained occult gastrointestinal blood loss from S. stercoralis. The parasite also can cause colonic inflammation that resembles ulcerative colitis but is more right-sided and strongly eosinophilic.33–35
While the parasite burden remains balanced, symptoms are minimal or absent. Immunosuppression or glucocorticoid administration upsets this balance. Previously asymptomatic, but chronically infested, patients develop fulminant, potentially fatal strongyloidiasis due to massive autoinfection.36,37 The mechanisms that permit massive autoinfection are unknown, but events that inhibit Th2-directed immune responses can release eosinophil-mediated control of the parasites. In addition, glucocorticoids can act directly on the parasites to increase the development of infective filariform larvae.38 Fulminant disseminated strongyloidiasis rarely complicates HIV and AIDS.39
Massive autoinfection produces disseminated fulminant strongyloidiasis. Migrating filariform larvae injure the intestinal mucosa and carry luminal bacteria into the bloodstream, resulting in polymicrobial sepsis with enteric organisms. Streptococcus bovis endocarditis or meningitis40 also can result. Numerous larvae migrating through the lungs cause pneumonitis, and worms can arrive in unusual locations such as the brain. Fulminant strongyloidiasis often is fatal.
Diagnosis
A recent survey of United States physicians-in-training demonstrated very poor ability to identify or even consider strongyloidiasis.41 Patients with chronic strongyloidiasis often are asymptomatic. Peripheral blood eosinophils may be elevated, but a normal eosinophil count does not argue against infestation with the parasite. Currently, the best method for detecting exposure is enzyme-linked immunosorbent assay (ELISA) for immunoglobulin (Ig) G antibodies against S. stercoralis. This assay is performed by the Centers for Disease Control and Prevention (CDC) in the United States and is 95% sensitive,42 sensitivity being highest for immigrants with prolonged exposure and lowest for returning visitors with lower-level recently acquired infestation.43
False-positive reactions can occur in patients exposed to other helminthic parasites,44 and serologic positivity can indicate prior exposure to S. stercoralis, not necessarily active infestation. Because chronic strongyloidiasis can remain subclinical and difficult to detect for decades, however, treatment of seropositive patients is warranted. Indeed, some argue that patients with only suspected strongyloidiasis, such as immigrants from endemic countries who have elevated eosinophil counts, should be treated empirically before glucocorticoid therapy.45
Active infestation can be diagnosed by finding rhabditiform larvae in direct smears of the stool, though this is an insensitive method. A 10-fold more sensitive technique is to spread stool on an agar plate and look for serpentine tracks left by migrating larvae.46 Intestinal biopsy is also an insensitive means of diagnosis.
Treatment
Chronic strongyloidiasis is best treated with one dose of ivermectin (200 µg/kg) given orally; this dose is used in both adult and pediatric patients. Ivermectin is better tolerated than thiabendazole. Ivermectin paralyzes the intestinal adult worms but not the larvae migrating through tissue, and therefore patients can develop recurrent infestation from migrating larvae; a repeat dose after two weeks helps to prevent this outcome. Successful treatment causes a fall in antibody titer by six months in most (about 90%) patients.42 Immunocompromised patients require repeat doses given 2, 15, and 16 days after the first dose.37
CAPILLARIA (PARACAPILLARIA) PHILIPPINENSIS
Capillariasis is acquired by eating raw fish that are infested with the parasite.47 The nematode causing capillariasis has been renamed from Capillaria philippinensis to Paracapillaria philippinensis,48 but by any name, it is deadly. The parasite replicates in the host, producing an ever-increasing number of intestinal worms. Patients develop protein-losing, sprue-like diarrhea with progressive emaciation and anasarca, which ultimately leads to death. Treatment is albendazole.
Epidemiology
The first known human case of capillariasis was reported in 1964. It remains a rare but deadly parasitic infestation. From 1965 through 1968, an epidemic in the rural Philippines involved 229 cases, with an overall mortality rate of 30%.49 As the name implies, Paracapillaria phillippinensis is endemic to the Philippines, but it also is endemic in Thailand and cases occur in Japan, Taiwan, Egypt, and Iran. Modern travel transports cases worldwide.50
Life Cycle
People become infested with the worm by eating raw or undercooked freshwater or brackish-water fish that contain the parasitic larvae. Some female adult P. philippinensis are larviparous, producing infective larvae instead of eggs. These larvae then mature in the small intestine and increase the parasite burden. This pathway of autoinfection permits a massive increase in parasite numbers. A rhesus monkey originally fed 27 larvae had more than 30,000 worms by 162 days of infection.51
Clinical Features and Pathophysiology
The progressive disease is believed to result from an ever-increasing number of poorly adapted intestinal parasites. In autopsy studies, the jejunal intestinal mucosa showed flattened, denuded villi with numerous plasma cells, lymphocytes, macrophages, and neutrophils infiltrating the lamina propria.47
Diagnosis
Diagnosis is made by finding eggs and larvae in stool specimens. No serologic tests for capillariasis are available. Symptomatic patients have detectable eggs in their stool. The eggs are easily confused with those of Trichuris trichiura, but T. trichiura eggs have prominent bipolar plugs that appear cut off in P. phillipinensis.47
HOOKWORMS (NECATOR AMERICANUS, ANCYLOSTOMA DUODENALE, AND ANCYLOSTOMA CANINUM)
Worldwide, an estimated 740 million people are infested with hookworm,17 usually by Necator americanus, Ancylostoma duodenale, or a mixture of the two. Hookworm is acquired by skin contact with contaminated soil. Moderate infestation contributes to iron deficiency. Hookworm should be suspected in patients with eosinophilia and iron-deficiency anemia. The dog and cat parasite Ancylostoma caninum is a cause of eosinophilic enteritis. Treatment is albendazole.
Necator americanus and Ancylostoma duodenale
Life Cycle
Infective third-stage hookworm larvae penetrate intact skin, such as between the toes of bare feet while walking on contaminated ground. Larvae migrate through the dermis to reach blood vessels. This migration can cause a pruritic, serpiginous rash, cutaneous larva migrans (Fig. 110-5). Ancylostoma braziliense normally infests dogs and cats, but it produces a similar rash during infective dermal wandering in humans and is the usual cause of cutaneous larva migrans. Larvae of N. americanus and A. duodenale enter blood vessels in the skin and migrate with venous flow through the right side of the heart to the lungs. A. duodenale larvae can arrest their migration and become dormant for many months before proceeding to the lungs.52 In the lungs, larvae penetrate the alveoli and enter the air spaces, after which they migrate up the pulmonary tree, are swallowed with saliva, and pass into the small intestine, where they mature. Patients also can acquire A. duodenale by directly ingesting larvae crawling on contaminated fresh vegetables. Adult worms develop large buccal cavities and graze on the intestinal mucosa, ingesting epithelial cells and blood (Figs. 110-6 and 110-7). Adults are about one centimeter long and can live for up to 14 years. Mature worms mate and lay eggs. Each female N. americanus lays about 10,000 eggs a day, and each female A. duodenale lays about 20,000 eggs a day. Eggs are deposited with feces in moist, shady soil, where they hatch to release larvae. The larvae molt twice after which they move to the soil surface and seek a suitable host.
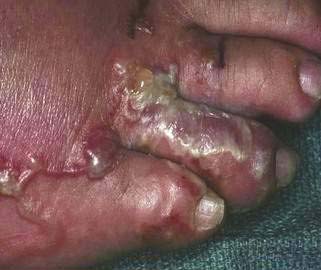
Figure 110-5. Serpiginous rash caused by hookworm larvae migrating through the dermis.
(Courtesy of the University of Iowa Department of Dermatology, Iowa City, Ia.)
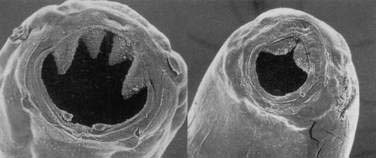
Figure 110-6. Scanning electron micrographic view of the buccal cavities of Ancylostoma duodenale (left) and Necator americanus (right).
(From Hotez PJ, Pritchard DI. Hookworm infection. Sci Am 1995; 272:70.)
Clinical Features and Pathophysiology
Light infestations with N. americanus and A. duodenale cause no symptoms.53 The major consequence of moderate and heavy hookworm infestation is iron deficiency. Adult worms feed on intestinal epithelial cells and blood. The closely related A. caninum (see later) secretes anticoagulant peptides that inhibit clotting factors54 and platelet aggregation,55 thereby preventing hemostasis and permitting the hematophagous parasites to feed on host blood. Intestinal blood loss is estimated to be 0.01 to 0.04 mL/day per adult N. americanus and 0.05 to 0.3 mL/day per adult A. duodenale.56 With a moderate number of worms, this blood loss becomes appreciable (Table 110-1). Iron deficiency results when iron loss outstrips iron absorption. The average North American diet is high in iron so anemia might not develop, and men with a diet high in iron (more than 20 mg/day) can tolerate up to 800 adult hookworms without developing anemia.
Table 110-1 A Comparison of Daily Physiologic Iron Losses and Iron Losses Due to Hookworm Infection in Women*
| CONDITION | IRON LOSS (MG/DAY) |
|---|---|
| Physiologic Losses | |
| Menstruation | 0.44 |
| Pregnancy | 2.14 |
| Lactation | 0.23 |
| Losses Due to Hookworm Infection | |
| Necator americanus (60-200 worms) | 1.10 |
| Ancylostoma duodenale (20-100 worms) | 2.30 |
* Losses shown are in addition to the basal iron loss of 0.72 mg/day.
Adapted from Stoltzfuss RJ, Dreyfuss ML, Chwaya HM, Albonico M. Hookworm control as a strategy to prevent iron deficiency. Nutr Rev 1997; 55:223-32.
Infestation with hookworm can modulate immune responses.57 Clinical trials are under way to determine if subclinical infestation with hookworm inhibits immune-mediated disease such as Crohn’s disease and asthma.58,59 Dose-ranging studies on healthy volunteers suggested that low-level hookworm infestation (10 larvae) is well tolerated.58
Diagnosis
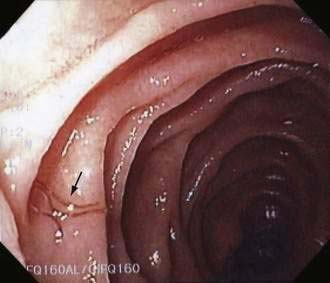
Hookworms can be visible endoscopically (Fig. 110-8),60 but diagnosis is made by identifying eggs on direct smears of formalin-fixed stool (see Fig. 110-3). Evaluation of three stool specimens obtained on separate days should permit diagnosis of hookworm,61 but light infestations can require concentration techniques. Eggs mature rapidly at room temperature and can hatch to release larvae. It is difficult to distinguish N. americanus eggs from those of A. duodenale simply by morphology.
Ancylostoma caninum
Clinical Features and Pathophysiology
A. caninum is a well-recognized cause of cutaneous larva migrans, a distinctive serpiginous rash caused by an abortive migration of the parasite in an unsupportive host. A. caninum also can cause eosinophilic enteritis, although not all eosinophilic enteritis is caused by this parasite (see Chapter 27). Patients with eosinophilic enteritis from A. caninum often are dog owners and present with colicky mid-abdominal pain and peripheral eosinophilia,62 but they do not recall having cutaneous larva migrans. Intestinal biopsies show high numbers (>45/high-power field) of mucosal eosinophils,63 and eosinophilic inflammation is most prevalent in distal small bowel. Unlike eosinophilic gastroenteritis, tissue eosinophilia is not present in the stomach. On endoscopy of the terminal ileum, patients might have scattered small superficial aphthous ulcers and mucosal hemorrhage.64 Serologic evidence suggests that A. caninum also may be a cause of abdominal pain without eosinophilia or eosinophilic enteritis.62
WHIPWORM (TRICHURIS TRICHIURA)
Epidemiology
An estimated 800 million people harbor T. trichiura. It occurs in temperate and tropical countries and remains prevalent in areas with suboptimal sanitation. In one equatorial Cameroon province, 97% of the school-age children had T. trichiura.65 Whipworm eggs are sensitive to desiccation, so prevalence is low in desert climates.
Life Cycle
T. trichiura has a simple life cycle. Colonization occurs by ingesting the parasite egg, each of which contains one developed larva. The eggs hatch in the intestine, and larvae migrate to the cecum, where they mature, mate, and lay eggs. This process takes about eight to 12 weeks. Adult worms are approximately three centimeters long and have a thin tapered anterior region so that the worm resembles a whip (Fig. 110-9, Video 110-2).66 A mature female worm lays about 20,000 eggs a day and can live for three years. Eggs are deposited with feces into the soil. Over the next two to six weeks, one larva develops within each egg, but the egg is not infective until it has fully embryonated. Therefore, T. trichiura does not multiply in the host and is not directly transmitted to other persons.
Clinical Features and Pathophysiology
Most persons with T. trichiura infestation have no symptoms attributable to the parasite. Most people in an endemic area are colonized by small numbers (less than 15) of worms and for them, the parasite is a commensal organism rather than a pathogen. Some people harbor hundreds or even thousands of worms,67 and they are the ones who develop symptoms68; this bimodal distribution of infestation persists after patients are treated and then become reinfected naturally, suggesting that unique host factors (genetic or behavioral) contribute to determining an individual patient’s worm burden.
Rectal prolapse can occur in children with extremely high numbers of T. trichiura worms.69 Some persons with numerous worms have mucoid diarrhea and occasional bleeding, a combination of symptoms called the Trichuris dysentery syndrome (TDS). Children with this condition have growth retardation,70 but studies attributing these symptoms to T. trichiura are complicated, because persons with TDS often are socioeconomically deprived and may be coinfected with other pathogens. Colonic biopsy specimens from children with TDS show few or no abnormalities compared with healthy local children,71 other than an increase in mast cells72 and in the number of cells that express TNF-α and calprotectin.73
A different but closely related species, Trichuris muris, infests mice. Mouse strains that react to the parasite with a strong Th2 response, characterized by production of interleukin (IL)-4, IL-5, and IL-13, are able to expel the worms, whereas strains that respond with a Th1 response (interferon [IFN]-γ) have difficulty expelling the worms.74 Blocking IL-4 makes resistant strains susceptible, and blocking IFN-γ makes susceptible strains resistant to chronic infestation with T. muris.75 The type of immune response developed by inbred mice to T. muris is an important factor in determining length and intensity of infestation. A similar response in humans might explain why some people repeatedly acquire heavy infestations whereas others carry only a few worms.
Diagnosis
Diagnosis is made by identifying T. trichiura eggs in stool specimens. Trichuris eggs are 23 µm by 50 µm and have characteristic plugs at each end (see Fig. 110-3).
Treatment
T. trichiura is treated with mebendazole 100 mg twice a day for three days; alternatively, patients can take albendazole 400 mg each day for three days. Heavily infested patients might require seven days of treatment.76 Single-dose treatment with albendazole is ineffective27 but one treatment with a combination of albendazole (400 mg) and ivermectin (200 µg/kg) appears quite effective, with cure rates of up to 80% and egg reduction rates of 94%.77,78
PINWORM (ENTEROBIUS VERMICULARIS)
Epidemiology
E. vermicularis is a quintessential intestinal parasite with no geographic constraints. It is transmissible by close contact with colonized persons. People have had pinworm for thousands of years, and before modern sanitation, colonization by pinworm probably was universal. E. vermicularis eggs were identified in a 10,000-year-old human coprolite found in Utah.79 The pinworm Enterobius gregorii, originally thought to be a separate species of pinworm,80,81 actually may be just a young adult form of E. vermicularis.82
Pinworm remains common in many areas, but it appears to be decreasing in prevalence. A survey of positive cellophane tape tests (see later) in New York City documented a sharp decline in positivity from 57 of 248 tests in 1971 to 17 of 165 in 1978 to 0 of 38 in 1986.83 Similar trends are reported from California.
Life Cycle
Eggs hatch in the duodenum, releasing larvae that molt twice as they mature and migrate to the cecum and ascending colon (Fig. 110-10, Video 110-3).84 The parasites are small: adult males measure 0.2 mm by 2 to 5 mm, and adult females measure 0.5 mm by 8 to 13 mm. After mating, gravid females migrate to the rectum. During the night, egg-laden females migrate out of the anal canal and onto the perianal skin. Each female deposits up to 17,000 eggs, which mature rapidly, becoming infective within six hours. Pinworm infestation typically causes perianal itching, and scratching gathers eggs onto the hands, promoting reinfection and transmission to others.
Clinical Features and Pathophysiology
E. vermicularis is an extremely well adapted parasite that produces no specific symptoms in the vast majority of colonized persons. Most symptoms are minor, such as pruritus ani and restless sleeping. Rarely, pinworm causes eosinophilia or eosinophilic enteritis.85
Vulvovaginitis is more common in girls with pinworm than in girls without this infection. Vulvovaginitis may be caused by migration of the worms into the introitus and genital tract. Dead worms and eggs encased in granulomas have been found in the cervix, endometrium, fallopian tubes, and peritoneum, attesting to the migratory effort of female worms.86 Ectopic enterobiasis is rare and causes no or very little overt pathology.
Infestation with E. vermicularis can influence mucosal immune responses. One case report described a 12-year-old girl with pinworm and apparently latent ulcerative colitis, who developed severe ulcerative colitis after treatment with pyrantel to remove the worms.87 While she was colonized with E. vermicularis, intestinal biopsies showed increased expression of mRNA for IL-4, transforming growth factor (TGF)-β, IL-10, and FOXP3 compared with biopsy specimens taken after anthelminthic treatment; these transcripts are associated with immune regulatory pathways that suppress inflammation.

 ) and female (
) and female ( ) whipworms.
) whipworms.