Chapter 22 Infections of the Luminal Digestive Tract
Introduction
Epidemiology
The prevalence, incidence, and etiology of luminal GI tract infections are influenced by many factors (Box 22.1). In the normal host, luminal GI infections generally occur randomly after exposure to a pathogen and are self-limited. Exposure may take many forms, such as occupational (day care centers), dietary, and environmental (contaminated water). Coexisting host factors may promote the clinical expression of infection and either attenuate or exacerbate the disease. For immunosuppressed patients, the incidence and severity of infection are linked to the cause and degree of the immunodeficiency state. Patients undergoing solid organ transplantation are at the highest risk of infection early on after transplantation because of profound medication-induced immunodeficiency. Latent infections become manifest during this period, and susceptibility to infections is greatest. Over time, however, as drug-induced immunosuppression is tapered, the incidence of infections decreases. For patients infected with human immunodeficiency virus (HIV), the incidence of GI infections increases markedly as immune function deteriorates, and the infection risk can be accurately stratified by the absolute CD4 lymphocyte count and HIV-1 RNA levels.1,2
Rarely, opportunistic infections have been observed in the apparently normal host, but in contrast to an immunodeficient patient, these infections are typically self-limited.3,4 Generally, the more severe the immunodeficiency state required for development of an opportunistic infection, the less likely the pathogen will be observed in the normal host. Although herpes simplex virus (HSV) is a well-recognized pathogen in otherwise healthy people,3 HSV esophagitis occurs most often in patients with some predisposing factor. In contrast, until the advent of transplantation, cytomegalovirus (CMV) was a rare pathogen, and its identification in any patient suggests some type of immune dysfunction.4,5 CMV is regarded as one of the most common opportunistic infections. This frequency of infection relates to the high prevalence of prior exposure to CMV, as reflected by seropositivity rates of more than 90% in developed countries,6 and to the fact that CMV disease generally occurs from recrudescence of latent infection during periods of profound immunosuppression.
Overall, the frequency of GI infections has been decreasing in immunosuppressed patients. Extensive research has defined the time course and spectrum of infections that complicate immunodeficiency states.7 Based on these observations, targeted preemptive antimicrobial prophylaxis has become the standard of care against many of these infections during periods of greatest vulnerability. Different classes of agents are used at varying time points depending on the infection risk and pathogens associated with the level of immune impairment. However, antimicrobial prophylaxis is associated with an increased risk for other infections and the development of drug resistance. The use of prophylaxis for Pneumocystis carinii infection in AIDS is associated with an increase in the prevalence of other opportunistic infections, such as viral disease, because patients are now living longer.8 The implementation of Candida prophylaxis in selected patients undergoing transplantation and the widespread use of oral antifungal therapies in AIDS has reduced the incidence of fungal infections in these settings but has been linked with drug resistance to Candida species.9
The use of highly active antiretroviral therapy for HIV-infected patients has drastically reduced the frequency of GI complications, including infections and neoplasms.10–12 More selective immunosuppressive therapy, such as with cyclosporine, has been beneficial in reducing the incidence of infections after transplantation.13 Methods for prophylaxis other than use of antimicrobials have also played a beneficial role in the reduction of opportunistic infections. In high-risk transplant patients, the use of CMV-seronegative organs and blood products for seronegative recipients, the use of leukocyte-depleted platelets for patients after bone marrow transplantation, and the administration of preemptive antiviral therapy all have reduced the incidence of CMV disease.14,15
With the increase in international travel for both business and pleasure, geography plays an increasingly important role in the prevalence of some GI infections. Pathogens are endemic in certain portions of the world and in specific regions within countries. In the United States, histoplasmosis is endemic in the Midwest and Mississippi Valley, and coccidioidomycosis is endemic in the Southwest. Mycobacterium tuberculosis (TB) is endemic in Third World countries and involvement of the GI tract is well recognized. Penicillium marneffei has been described as a pathogen more recently and appears to be limited geographically to Southeast Asia.16 Traveler’s diarrhea, usually caused by enteropathogenic Escherichia coli, is characteristically seen with travel to Mexico and other developing countries.17
Pathogenesis
The pathogenic mechanisms of luminal GI infections are (1) exposure to a pathogen, (2) reactivation of prior infection (recrudescence), (3) overgrowth of a commensal organism, and (4) local spread or dissemination. The specific organ involved with any infectious process, other than local spread, is dictated by the organ-specific tropism of the infecting pathogen. Candida and HSV almost exclusively infect squamous epithelium, whereas Campylobacter jejuni and Shigella species are colonic pathogens. Inherent to any discussion of pathogenesis is the issue of host-related factors. Numerous nonspecific and immune-based defense mechanisms both prevent and attenuate GI infections.18 These defenses may be altered by disease, by medications, or as a part of the aging process. Saliva provides an effective physical barrier because of its physical properties, and immunoglobulins that are present in saliva and in intestinal secretions provide an important early line of defense. Gastric acid is a barrier to enteropathogens, and hypochlorhydria has been shown to be a risk factor for the development of cholera and other GI infections.19 GI motility moves ingested pathogens through the gut and prevents stasis, which can lead to bacterial overgrowth. Inherent antibacterial proteins secreted by Paneth cells, termed defensins, seem to play a key role in the host response to bacterial infections of the gut.20
The mucosal immune system is composed of inflammatory cells, most notably T cells.18 After exposure to a foreign antigen, these cells differentiate into helper or cytotoxic cells depending on whether the cells express the CD4 or CD8 receptor. The release of cytokines by these cells plays a key role in limiting infection but can also result in tissue damage. The critical role of the mucosal immune system in preventing and controlling infections is best shown by the array and severity of luminal GI infections that occur in AIDS, in which a progressive loss of CD4 lymphocytes from both the systemic circulation and the mucosal-based immune system occurs.21 Loss of these cells predisposes to small intestinal infections by opportunistic infections such as cryptosporidiosis and microsporidiosis. Likewise, lymphocyte dysfunction, either medication-induced or as part of an immunodeficiency state, predisposes to symptomatic primary infection or recrudescence (e.g., CMV).
Bacterial esophagitis is a polymicrobial infection consisting of oral flora, particularly gram-positive organisms, including Streptococcus viridans, staphylococci, and other bacilli. This rare cause of esophagitis occurs under conditions of absolute granulocytopenia or severely impaired granulocyte function in which these commensal bacteria invade mucosa that has been damaged from reflux disease, from radiation therapy, or from chemotherapy, leading to an active local infection and potential dissemination.22 GI infections may result secondarily from active disease in adjacent organs. Esophageal disease may be caused by contiguously infected mediastinal lymph nodes or pulmonary parenchymal infection23 and by spread of infection via a draining fistula or obstructed lymphatics, resulting in tracheoesophageal fistula.24 Widespread lymphohematogenous dissemination of opportunistic infections causes either diffuse or focal disease anywhere in the gut; this process is generally limited to only the most severely immunocompromised patients.25,26
Most intestinal infections result in tissue inflammation of varying degrees. Local upregulation of cytokines plays a central role in the local immune response to the pathogen but may also cause tissue injury. CMV esophagitis is associated with high mucosal concentrations of the proinflammatory cytokine tumor necrosis factor.27 Toxin production and virulence factors play a key role in the clinical expression and tissue damage caused by many GI infections, especially bacterial infections.28
Clinical and Endoscopic Features
Esophagus
Clinical Features
The most prevalent cause of esophageal infection in both the normal host and the immunosuppressed patient is Candida followed by the herpesviruses.5,29,30 CMV occurs more commonly in patients with AIDS, whereas HSV is more often observed in the normal host and non–HIV-infected immunosuppressed patients. Odynophagia is the characteristic symptom of esophageal infection, and infections resulting in esophageal ulceration almost uniformly cause odynophagia.5,31 Although less common, dysphagia may be observed with esophageal infections, especially Candida esophagitis, or may represent esophageal obstruction or dysmotility from the infection or its sequelae. Bleeding is generally observed only when there is ulceration, and although generally mild, it can be severe if there is an associated coagulopathy. Pulmonary symptoms may predominate when there is fistula formation to the tracheobronchial tree or coexistent pulmonary involvement. Patients with AIDS often have multiple coexisting esophageal disorders, which complicate management further.5,32
Physical examination, particularly of the oropharynx, may be helpful in suggesting the diagnosis of esophageal infection. Approximately two-thirds of patients with AIDS and esophageal candidiasis have oral candidiasis (thrush).33 In other immunocompromised patients, oropharyngeal candidiasis is also commonly associated with esophageal candidiasis.5 Thrush may be absent, however, if antifungal therapy, such as nystatin, is currently administered. The presence of oropharyngeal candidiasis does not prove that Candida is the only cause of symptoms, and the absence of oropharyngeal candidiasis does not exclude Candida esophagitis. Patients with chronic mucocutaneous candidiasis may have fungal involvement of various mucous membranes, hair, nails, and skin and have a history of adrenal or parathyroid dysfunction. Coexistent oropharyngeal ulceration is common in patients with HSV esophagitis but is infrequent in patients with CMV esophagitis or other systemic infections.34,35
After Candida species, herpesviruses are the most frequent infectious agents that cause esophagitis. After transplantation, HSV and CMV occur with equal frequency as causes of esophagitis,5 whereas in patients with AIDS, HSV esophagitis is uncommon and far less frequent than CMV. In a study of 100 HIV-infected patients with esophageal ulcer, HSV was found in only 9 (in 4, it was a copathogen with CMV).30 HSV esophageal infection commonly manifests with the sudden onset of severe odynophagia, heartburn, or chest pain.3,34 Autopsy studies suggest that esophageal symptoms may be absent. Herpes labialis (i.e., cold sores) and oropharyngeal ulcers may coexist, antedate, or develop during the esophageal infection, whereas skin infection is rare.5 Numerous systemic manifestations, including low-grade fever or upper respiratory symptoms, may precede the onset of esophageal symptoms. In untreated immunocompetent persons, spontaneous resolution of HSV esophageal infection occurs within 2 weeks of the onset of symptoms. Rarely, bleeding is the initial presentation and may be observed in the absence of esophageal complaints.
Odynophagia is almost uniformly present and is characteristically severe with CMV esophagitis. Chest pain, weight loss, and fever may be reported. The onset of symptoms is often more subacute than the acute presentation of HSV. A prior or coexistent diagnosis of CMV infection in other organs (e.g., retinitis or colitis) is frequent. Although rare in transplant patients, retinitis may be observed in approximately 15% of AIDS patients at the time of diagnosis of GI disease.35 The frequency of esophageal involvement with other pathogens is rare. Bacterial esophagitis has been observed in patients with severe neutropenia, usually patients with hematologic malignancies, but occasionally it is observed after bone marrow transplantation,36 diabetic ketoacidosis,37 or steroid therapy. The presentation is similar to the presentation with other infection agents.
Bacteria reported to involve the esophagus include Brucella,38 Actinomyces,39 Nocardia,40 and Bartonella henselae.41 The symptoms of esophageal TB depend on the degree and type of involvement.24,42,43 Systemic symptoms of fever and weight loss are common. Pulmonary complaints often predominate because of a fistula to the trachea, bronchus, or pleural space. Dysphagia may be prominent with the formation of long strictures or traction diverticula resulting from the fibrotic response. Upper GI hemorrhage caused by esophageal ulcers or tuberculous arterioesophageal fistulas may be the primary manifestation. Bleeding caused by extensive mucosal disease has been described in an AIDS patient with esophageal Mycobacterium avium complex (MAC).44,45 Fungi other than Candida species and parasitic diseases have rarely been reported to involve the esophagus.46–51
Barium radiography plays a minor role in the diagnosis of esophageal infection. In any patient, the presence of severe odynophagia limits the ability to drink barium, hampering the adequacy of the examination. Although specific barium esophagogram findings may be more typical for certain disorders,52 given the potential overlap, many of the findings are nonspecific, and endoscopy with biopsy is generally indicated. The wide spectrum of causes coupled with the specific antimicrobial regimens that are required necessitates a definitive diagnosis rather than empiric antimicrobial therapy. Nevertheless, a sinus tract or fistulous connection to the bronchial tree or mediastinum at the level of the hilum is highly suggestive of TB, although it also may be the result of malignancy. An esophageal neoplasm may be mimicked by an ulcerated tuberculous granulomatous mass or CMV ulcer.42,53 Chest radiography or computed tomography (CT) scan of the chest may support the diagnosis of TB.
Endoscopic Features
The characteristics of the esophageal lesions provide very important diagnostic clues. The location, size, and appearance of all endoscopic abnormalities should be documented because these features form the basis of the differential diagnosis and are useful for comparison on follow-up endoscopic examinations. The differential diagnosis of the lesion dictates how lesions should be sampled and what recommendations for diagnostic testing should be made on the biopsy or cytologic specimens (discussed later). Serologic testing plays no significant role in the diagnosis of acute infectious esophagitis. Endoscopic examination of the esophagus is the most sensitive and specific method for diagnosing esophageal candidiasis (Table 22.1).
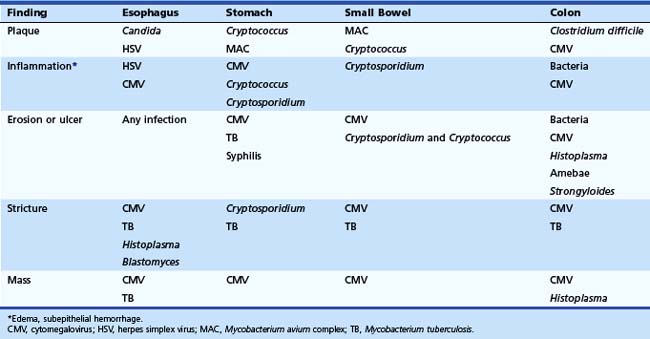
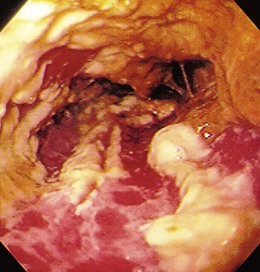
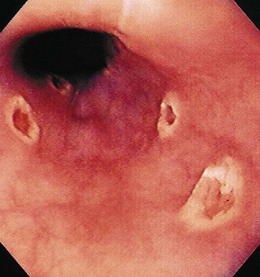
The gross endoscopic appearance of Candida esophagitis is pathognomonic (Fig. 22.1) and may be graded according to published criteria.54 A large, well-circumscribed ulceration should not be attributed to Candida. The endoscopic characteristics of HSV esophagitis reflect the pathologic changes. HSV esophagitis appears as discrete, usually small (<1 cm), well-circumscribed shallow ulcers; a diffuse erosive esophagitis; or rarely vesicles (Fig. 22.2).5,55 Small, scattered lesions covered with exudate mimic esophageal candidiasis. Deep ulcers, as seen with CMV, are very rare. CMV esophagitis is characteristically associated with one or more ulcerations that can be quite striking in patients with AIDS. Nevertheless, as with other infections, variability has been reported with appearances ranging from multiple shallow ulcers, to solitary giant ulcers, to a diffuse superficial esophagitis (Fig. 22.3).56 Although serologic testing is not helpful because of the high rate of prior exposure to CMV, the absence of CMV DNA or antigenemia in the blood would suggest an alternative diagnosis. Esophageal TB can manifest with a fistula to the tracheobronchial tree easily visualized endoscopically and rarely as an ulcer or mass lesion resembling a neoplasm. In normal hosts from endemic areas in South America, Trypanosoma cruzi may involve the myenteric plexus of the esophagus resulting in Chagas’ disease and an appearance that is indistinguishable clinically, radiographically, manometrically, and endoscopically from idiopathic achalasia.57 This diagnosis may be established by antibody testing. The endoscopic appearances of other rare infections have been described in case reports and resemble other infections.
Stomach
Clinical Features
Symptomatic gastric infections are much less prevalent than infections of the esophagus. The primary gastric pathogens are Helicobacter pylori and CMV; parasites and mycobacteria are also reported but are uncommon.58 Because of the relative infrequency of gastric infections, understanding of the presentation and endoscopic findings of most of these infections is based on case reports or small series. With the exception of H. pylori, most infections of the stomach occur in the setting of an immunodeficiency state. Gastric infections are typically manifested by upper abdominal pain that is generally steady and may radiate to the back. Associated symptoms may include nausea with or without vomiting; vomiting may be prominent when mucosal infection is severe. Infrequently, nausea alone in the absence of abdominal pain may be observed. Fever and weight loss are variable. Diarrhea may be the prominent symptom if the infecting pathogen also involves the small bowel (e.g., Cryptosporidium
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree