Chapter 11 Infections in liver, biliary, and pancreatic surgery
Infection
Infection is defined as a pathologic state resulting from invasion of the body by pathogenic microorganisms—bacteria, yeast, parasites, or viruses. These agents can challenge host defense mechanisms because of either an infectious organism’s virulence or a weakness in host defenses. In most instances, infection results when normal host defense mechanisms fail to prevent tissue penetration and the subsequent local propagation and/or systemic dissemination of the injurious agent. All infections can be complicated by sepsis, which is the invasion of a micoorganism or its toxins into the bloodstream, coupled with the host response to that invasion. More specifically, sepsis is a systemic inflammatory response syndrome caused by infection (American College of Chest Physicians/Society of Critical Care Medicine Consensus Conference Committee, 1992). When sepsis remains untreated, altered host inflammatory and coagulation cascades—intracellular homeostasis, cellular hypoxia, endothelial dysfunction, apoptosis, and eventually cardiovascular collapse—lead to multiorgan failure and death. Despite recent advances in treatment (Rivers et al, 2001), the incidence of sepsis and septic shock has been increasing (Angus et al, 2001). Furthermore, the associated mortality rate remains at 30% among all septic patients, with rates increasing to 85% in the setting of multiple organ failure (Angus et al, 2001).
Patients undergoing hepatopancreatobiliary surgery are at particular risk for infection and sepsis because of the severity of their disease process, often complicated by comorbid medical conditions and exacerbated by the stress of the surgical treatment itself. Hepatopancreatobiliary diseases that place patients at particular risk include infected necrotizing pancreatitis and sepsis associated with uncontrolled leakage from pancreatic–enteric anastamosis following pancreatoduodenectomy (Howard et al, 2007; Adams, 2009). This chapter reviews the applicable host defense mechanisms as well as their alteration by hepatopancreatobiliary diseases and operations.
Host Defenses
The host defense mechanisms preventing the entry of infectious organisms into the liver, biliary tract, and pancreas can be organized into three broad categories (Krige & Bornman, 2000): physical, chemical, and immunologic (Table 11.1). Although these mechanisms are distinct, significant overlap occurs. Furthermore, optimal defense often requires coordination of all three systems.
Table 11.1 Hepatobiliary and Pancreatic Host Defense Mechanisms
| Hepatobiliary | Pancreatic | |
|---|---|---|
| Physical | Biliary sphincter | Pancreatic sphincter |
| Hepatic tight junctions | Pancreatic tight junctions | |
| Bile flow | Pancreatic juice flow | |
| Mucus | Mucus | |
| Cilia | Cilia | |
| Chemical | Bile salts | Pancreatic fluid |
| Immunologic | Kupffer cells | — |
| Immunoglobulin A | Immunoglobulin A | |
| Fibronectin | — | |
| Complement | Complement | |
| CD40 receptor | CD40 receptor | |
| Blood supply | Blood supply |
Infectious microorganisms can enter the liver either hematogenously or retrograde via the biliary ductal system. The most common hematogenous route of infectious entry is the portal venous system. Under normal circumstances, the portal system drains all abdominal gastrointestinal venous blood directly into the liver before it enters the systemic circulation. As a result, portal venous blood typically contains enteric pathogens and toxins, which are cleared and neutralized by the liver (see Chapter 9). This activity is primarily regulated by Kupffer cells, which act as the resident hepatic macrophages. Consequently, Kupffer cells are not only important determinants of normal hepatic physiology and hemostasis but also of any inflammatory response. The liver therefore acts as the ultimate barrier to infection for all gastrointestinal tract and peritoneal cavity microorganisms attempting to access the systemic circulation. Perturbation of this flow, in particular portal venous obstruction, may occur from causes that are presinusoidal (portal vein thrombosis, surgery, trauma, cancer), sinusoidal (hepatitis, cirrhosis), or postsinusoidal (Budd-Chiari syndrome, right heart failure). Such obstructions often lead to portal hypertension with subsequent portosystemic shunting (see Chapter 70A). This new access to the systemic circulation allows a bypass of the liver’s defenses against enteric pathogens and toxins, thereby increasing the risk of bacteremia and sepsis. Although less common, direct hematogenous spread of microorganisms to the liver may also occur via the hepatic arterial system (e.g., bacterial endocarditis, intravenous drug use).
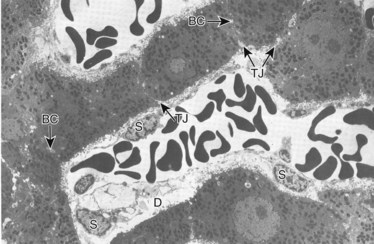
Under normal circumstances, an intact sphincter of Oddi provides an effective mechanical barrier that prevents bacteria and other enteric organisms from entering the liver (retrograde) via the biliary system (see Chapter 1B). Ascending infection from the intestine can access the biliary tree when the sphincter mechanism is destroyed, endoscopically or surgically; bypassed, as in a biliary-enteric anastomosis; or violated, such as via a transsphincteric stent. The biliary system can also be directly contaminated by percutaneous access via either the transhepatic route or with operatively placed drains (e.g., T-tubes). Fortunately, the presence of bacteria within bile does not uniformly result in an ascending biliary infection, because the main physical barrier to biliary sepsis remains: intrahepatic tight junctions (Fig. 11.1), which form a barrier between bile canaliculi and hepatic sinusoids and act to keep bile separated from the bloodstream. Under pathologic conditions, mixing of these two compartments can result in seeding of biliary organisms into the hepatic blood supply. Another physical hepatic defense mechanism is the flow of bile itself. Bile is produced by hepatocytes and excreted into bile canaliculi, which then coalesce to form larger ductules, major sectoral ducts, and ultimately extrahepatic biliary ducts. Bile flow out of the liver helps prevent ascending infection by its mechanical flushing action; however, interruption of flow via obstruction or stasis reduces this protective mechanism (see Chapter 43).
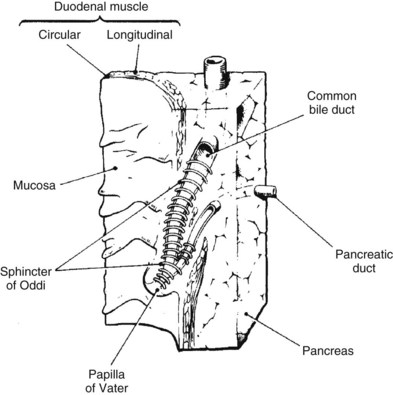
As previously discussed, the main physical host defense against the reflux of enteric contents, and adherent bacteria, into the biliary system is the biliary sphincter within the sphincter of Oddi (Fig. 11.2; see Chapter 1A, Chapter 1B ). Similar to the liver, bile flow also assists the biliary system by flushing and helping prevent free reflux of duodenal contents into the extrahepatic ducts. The bile duct epithelium within the ductules has a single cilium, which facilitates propulsion of bile toward the extrahepatic biliary ducts (Gilroy et al, 1995; Itoshima et al, 1977). Bile flow is further propelled toward the duodenum by gallbladder contraction. A final physical barrier within the biliary tract is mucus produced by the epithelial cells throughout the extrahepatic bile ducts. Mucus promotes distal flow by preventing adherence of bacteria and debris to the epithelial cell membrane (see Chapter 7).
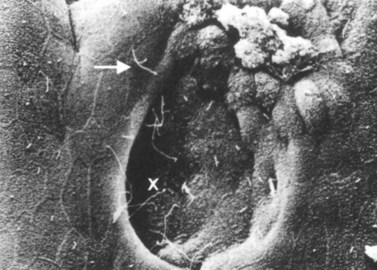
Similar to the biliary system, the main physical host defense mechanism for the pancreas is the pancreatic sphincter within the sphincter of Oddi. Although a true anatomic pancreatic sphincter is present in only one third of patients, all normal glands have a pressure zone at the end of the pancreatic duct that relaxes in response to secretin (Carr-Locke et al, 1985). This pressure zone prevents reflux of enteric contents into the pancreatic ductal system. Similar to the liver, the pancreas also contains paracellular seals or tight junctions between acinar and intralobular ductal epithelial cells. The integrity of the pancreatic tight junctions can be compromised, however, in experimental states of inflammation or pancreatitis, and possibly when ductal pressures are elevated (Fallon et al, 1995; Schmitt et al, 2004). The pancreatic ductal system is also aided by the flow of pancreatic duct fluid (flushing) to prevent reflux of duodenal contents. To facilitate this flow, the epithelium in the secondary ducts has a single cilium (Fig. 11.3) that propels secretion into the main pancreatic ductal system. Similar to the biliary system, the pancreatic ductal epithelium also secretes mucus, which acts as a barrier to the adherence of bacteria and debris (Moniaux et al, 2004; Trede & Carter, 1997).
Chemical host defense mechanisms also exist in the hepatic and pancreatobiliary systems. Bile salts within the biliary ducts have been shown to have bacteriostatic and bactericidal properties (Stewart et al, 1986; Sung et al, 1993). The maintenance of intestinal flora by bile salts is also crucial to host defenses, because bile salts have the ability to suppress pathogenic bacteria that threaten the integrity of the mucosal barrier, and they promote translocation of bacteria into the portal circulation (Bradfield, 1974; Clements et al, 1993; Deitch et al, 1990). Conditions of either bile salt loss or administration of prolonged courses of antibiotics may interfere with the balance of the intestinal flora and weaken this important host defense. Bile salts have also been shown to have antifungal effects, particularly against Candida albicans (Marshall et al, 1987). Furthermore, their trophic impact on epithelial cells increases both their integrity and ability to withstand insult from infectious pathogens (Stewart et al, 1986). Finally, bile salts posses an antiendotoxin effect (Stewart et al, 1986).
Pancreatic ductal fluid also contains inherent antibacterial properties (Rubinstein et al, 1985) independent of its enzymatic activity. Data suggest this fluid is bactericidal against multiple bacteria, including Escherichia coli, Shigella and Salmonella species, and Klebsiella pneumoniae (Kruszewska et al, 2004). Pancreatic fluid is also bacteriostatic against Staphylococcus aureus, S. epidermidis, and Pseudomonas aeruginosa; however, Bacteroides fragilis and Streptococcus faecalis are resistant to effects of pancreatic ductal fluid (Rubinstein et al, 1985). According to studies in swine, the antibacterial activity of pancreatic fluid appears to be independent of digestive enzymes. It is highest before eating and lowest during digestion of food, suggesting regulation through alternative signaling pathways (Holowachuk et al, 2004). By virtue of its antibacterial properties, pancreatic ductal fluid also regulates the colonization of enteric bacteria throughout the intestine by allowing the flora to maintain a normal homeostatic balance (Kruszewska et al, 2004).
In addition to its antibacterial effects, pancreatic ductal fluid appears to enhance the inherent action of certain bactericidal antibiotics (e.g., quinolones, trimethoprim, chloramphenicol, tetracycline) in studies comparing microbiologic media with those containing pancreatic ductal fluid (Mett et al, 1984). This enhanced activity has been shown toward many species of bacteria, including E. coli, Salmonella, Serratia, Proteus, and Staphylococcus. An unidentified, low-molecular-weight factor (independent of complement or lysozyme) in the pancreatic ductal fluid has been implicated (Mett et al, 1984). Although this factor is unknown, reproducible data show enhanced antibacterial activity in combination with commonly employed antibiotics, such as mezlocillin, ceftriaxone, gentamicin, ciprofloxacin, ofloxacin, and imipenem (Minelli et al, 1996). The relative degree of enhanced activity from pancreatic juice also depends on the specific bacteria (Minelli et al, 1996). Pancreatic ductal fluid contains fungistatic properties, in particular toward C. albicans (Kruszewska et al, 2004; Rubinstein et al, 1985), as well as growth factors (e.g., epidermal growth factor) that have a trophic effect on pancreatic epithelial cells. This helps preserve their integrity to pathogens (Alvarez et al, 1997). It is unknown whether pancreatic ductal fluid offers any antiendotoxin effects.
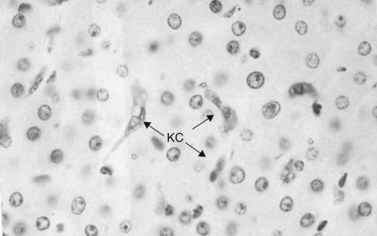
Immunologic host defense mechanisms also exist in the hepatic and pancreatobiliary systems. Hepatic cellular defense is provided by a large phagocyte population termed Kupffer cells (Fig. 11.4) as well as a plethora of other immunologically active cells (see Chapters 9 and 10). Kupffer cells are strategically located in the sinusoids of the liver, where they filter endotoxins from the gut, phagocytose bacteria, regulate sinusoidal vascular responsiveness, process and present antigens to T cells, and secrete cytokines, such as interleukins (ILs), interferons (INFs), and tumor necrosis factor (TNF) (Laskin, 1990). Kupffer cells represent one third of all nonparenchymal liver cells and 85% of all mononuclear phagocytes in the body (Kimmings et al, 1995; Wang et al, 1993). With the exception of circulating mononuclear cells, there is no evidence of a functionally equivalent resident phagocyte population in the pancreas.
Humoral defenses also exist within the hepatic and pancreatobiliary systems. Immunoglobulin (IG) A is secreted into the bile from the gallbladder and intrahepatic/extrahepatic biliary epithelium and plays an important role in barrier protection from infectious pathogens (Emmrich et al, 1998; Scott-Conner & Grogan, 1994) and is also present in pancreatic ductal fluid. The concentration of IgA is inversely related to pancreatic ductal fluid volume (Ohshio et al, 2001). In disease states characterized by exocrine insufficiency (e.g., chronic pancreatitis), a higher concentration of IgA is also observed in the remaining human pancreatic ductal fluid (Emmrich et al, 1998).
Other immunologic molecules in the bile include fibronectin, which opsonizes pathogens and facilitates biliary epithelial defense against bacteria (Wilton et al, 1987). Complement—in particular, C3, C4, and factor B—is also present and functionally active in the bile at levels similar to serum (Sumiyoshi et al, 1997). As expected, clinical conditions of C3 deficiency are associated with increased susceptibility to infection in the biliary system and elsewhere (Homann et al, 1997). Complement is also present in pancreatic ductal fluid (Andoh et al, 1996; Sumiyoshi et al, 1997). Specifically, C3, C4, and factor B are synthesized in the pancreatic ductal epithelium and secreted in response to proinflammatory cytokines IL-1β, TNF-α, and INF-γ (Andoh et al, 1996; Sumiyoshi et al, 1997). These cytokines act as the primary drivers for sepsis (Cohen, 2002).
The biliary and pancreatic epithelium also may have some inherent immunologic ability. Normally, the CD40 receptor is expressed on regenerating or inflamed bile duct epithelium. CD40-CD40 ligand interactions may be responsible for immunity to certain pathogens. CD40 is also expressed on pancreatic ductal epithelial cells and may play a similar role (Vosters et al, 2004). Patients with deficient CD40 ligand (X-linked immunodeficiency with hyper-IgM) show a higher incidence of hepatopancreatobiliary infections, inflammation, and carcinoma (Hayward et al, 1997).
Host Defenses Challenged by Underlying Hepatopancreatobiliary Conditions in Surgical Patients
Liver
Underlying hepatic conditions that may challenge host defenses in a surgical patient include hepatitis, cirrhosis, liver failure, portal hypertension, and hepatocellular toxin–induced dysfunction (see Chapters 64 and 70A). Hepatitis, cirrhosis, and liver failure all represent relative degrees of hepatic dysfunction, insufficiency, and compromise. As hepatic function deteriorates and ultimately leads to liver failure, physical, chemical, and immunologic hepatic host defenses also become compromised. With decreased bile production capacity, the physical defense of flushing bile flow is less efficient. The effect of primary liver cell dysfunction on hepatic tight junction integrity is unknown, but these junctions are altered in biliary obstructive causes of liver dysfunction. Bile salt deficiency associated with liver dysfunction leads to inadequate chemical defenses of bile salt–mediated antibacterial and antiendotoxin effects, as well as epithelial tropism, which results in reduced integrity of the epithelial barrier. The bacteriologic flora of the intestine is also altered, which may increase the quantity and change the type of bacteria that translocate into the portal system (Clements et al, 1993; Deitch et al, 1990). Liver dysfunction also leads to primary and secondary decreases in Kupffer cell function and to depressed synthesis of immunologically active proteins, which impairs cellular and humoral immunologic defense. Finally, cellular immunity may also be affected by the altered number and type of T cells present within the liver in hepatitis and cirrhosis (Deignan et al, 2002).
Portal hypertension is responsible for secondary decreases in Kupffer cell function. Resultant portosystemic shunting in patients with cirrhosis may compromise reticuloendothelial cell function by allowing enteric pathogens to be moved away from Kupffer cells. Portal hypertension therefore contributes to an increased risk of infection in animals with liver dysfunction (Basista et al, 1994). Local blood flow is also substantially altered in portal hypertension and cirrhosis, as intrahepatic sinusoidal resistance is increased. Sinusoidal responsiveness to endogenous vasodilators is depressed, whereas responsiveness to endogenous vasoconstrictors is increased (Birney et al, 2003). Endothelin and nitric oxide are two significant endogenous mediators of stellate cell contractility that modulate the local intrahepatic resistance in portal hypertension (Rockey, 2001).
Biliary System
The most well-studied underlying biliary condition that can interfere with host defenses in a surgical patient is biliary obstruction (see Chapter 7). This condition may occur from causes that are intrinsic (tumors, stones, strictures), extrinsic (pancreatitis, tumors), or functional (sphincter of Oddi dysfunction). The increased pressure within the biliary system as a consequence of biliary obstruction leads to weakened hepatic tight junctions. This effect is even more dramatic when pressure increases rapidly, such as with biliary tract manipulation (Raper et al, 1989). Leaky tight junctions result in bile reflux into the hepatic sinusoids (i.e., cholangiovenous reflux). Other studies suggest that cholangiovenous reflux may occur directly from bile ductules, through the spaces of Mall and Disse (see Fig. 11.1), into the hepatic sinusoids. This pathway measures 1.7 to 10 µm and offers less resistance to bacteria and toxins than higher resistance pathways such as hepatic tight junctions, biliary canaliculi, or hepatocytes (Stewart et al, 1988). During states of biliary obstruction, the reduced flow of bile also diminishes the physical barrier of the flushing action of bile. Obstruction causes decreased bile salt delivery to the intestine, resulting in reduced bile salt–mediated bacteriostatic and bactericidal activity and therefore colonic bacterial overgrowth. This overgrowth disturbs the protective bacterial flora and promotes local inflammation, injury, and subsequent increased rates of translocation into the portal circulation (Clements et al, 1993; Deitch et al, 1990). The increased bacterial load is not adequately cleared in the liver, allowing entry into the systemic circulation (Bradfield, 1974).
In addition to the direct local effect, jaundice associated with biliary obstruction can substantially alter host defenses. It causes depressed Kupffer cell function by reducing clearance capacity. This results in an increased bacterial load, which overwhelms the hepatic sinusoids and increases bacterial entry into the bloodstream (Clements et al, 1993; Ding et al, 1992; Katz et al, 1991; Megison et al, 1991; Pain et al, 1987).
The cause of Kupffer cell dysfunction in biliary obstruction and jaundice is unclear. One plausible theory relates increased bacterial translocation from the ileum and colon into the portal venous system, thereby directly overwhelming Kupffer cell clearance. Other theories include direct effects of bile salts on Kupffer cell membranes (Takiguchi & Koga, 1988), ineffective opsonins (e.g., fibronectin), decreased sinusoidal size and blood flow, and reduced major histocompatibility complex expression (Clements et al, 1994a, 1994b; Ding et al, 1992; Takiguchi & Koga, 1988).
The role of endotoxins on Kupffer cell function is also poorly understood, and animal studies suggest that biliary obstruction and portal vein endotoxinemia may generate oxygen free radicals in the liver that reduce the ability of Kupffer cells to clear toxins (Ogura et al, 1996; Takahashi et al, 1996). The use of oxygen free-radical scavengers, such as coenzyme Q10 or styrene-co-maleic acid superoxide dismutase in animal models, has been shown to interrupt the detrimental effects of endotoxin on Kupffer cell clearance and hepatic immunologic reserve (Ogura et al, 1996; Takahashi et al, 1996). Biliary obstruction results in less IgA production by the liver and lowers bile IgA levels. Endoscopic drainage of patients with biliary obstruction resolves this IgA deficiency in the bile (Sung et al, 1995); however, prolonged biliary obstruction eventually leads to hepatocyte dysfunction and necrosis, impairing liver synthetic function. Many proteins with immunologic importance are produced less efficiently, resulting in an increased susceptibility to infection.
Clinical relevance of the host challenge associated with biliary obstruction is evident in the higher probability of morbidity and mortality in patients with obstructive jaundice who undergo invasive procedures (Blamey et al, 1983; Pitt et al, 1981; Povoski et al, 1999; Schmidt et al, 2004).
Numerous controlled studies have suggested that preoperative external biliary drainage is associated with increased infectious morbidity. This is likely related to bile loss and the risk of infection and inflammation with catheterization of the biliary tree (Hatfield et al, 1982; Hochwald et al, 1999; Kimmings et al, 2000; McPherson et al, 1984; Pitt et al, 1985; Povoski et al, 1999; Smith et al, 1984; Sohn et al, 2000; van der Gaag et al, 2009). Internal stenting offers a theoretic benefit, with avoidance of external catheters and a return of bile to the gastrointestinal tract (eneterohepatic cycle). Unfortunately, multiple retrospective studies have reported mixed results (Trede & Schwall, 1988; Sohn et al, 2000; Schmidt et al, 2004), and four prospective randomized controlled trials provide conflicting data. These trials typically include patients undergoing pancreaticoduodenectomy (periampullary or pancreatic head adenocarcinoma), randomized to either preoperative endoscopic biliary drainage or surgery without drainage (see Chapters 50B, 50C, 62A, 90B, and 90C). Because of varied findings, however, the authors’ conclusions have ranged from recommending routine preoperative endoscopic biliary drainage for patients with biliary obstruction (Lygidakis et al, 1987) to suggesting that although liver function improves with internal stenting, there is no difference in infectious outcomes (Lai et al, 1994). The most recent and methodologically sound trial reports that routine preoperative biliary drainage actually increases the rate of complications related to surgery (van der Gaag et al, 2010). Finally, a Cochrane review, as well as a meta-analysis, concluded no positive effect was seen from preoperative endoscopic biliary stenting in obstructed patients (Saleh et al, 2002; Wang et al, 2008).
Differences in the perioperative risk of pancreatectomy and major partial hepatectomy in jaundiced patients must be recognized. Contrary to the results in pancreas, some evidence shows improved outcome with decompressing the future liver remnant in patients with proximal biliary tumors (see Chapters 50C and 90C).
Theoretically, internal biliary stenting is advantageous over externalized stents, because it avoids the loss of bile and may reduce bacterial contamination. In animal models of obstructive jaundice, biliary decompression via internal stenting promotes Kupffer cell recovery (Clements et al, 1996), as evidenced by increased cell clearance capacity and normalization of endotoxin and anti–core glycolipid antibody concentrations (Greve et al, 1992). It has been suggested that the inconsistent results of clinical trials may be due to inadequate time allowed for liver recovery. The duration of biliary decompression needed to affect these parameters is unknown (van der Gaag et al, 2009
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree