Infected Field Hernia Repair
Emanuele Lo Menzo
Adrian Park
More than two million laparotomies are performed annually in the United States, and between 2% and 20% will result in incisional hernias. Typically, hernias become clinically apparent in the first 5 years after laparotomy.
Since the early description of temporary abdominal wall closures, their utilization has exponentially increased, particularly in trauma surgery. Their wide application inevitably leads to increased incidence of large skin-grafted ventral hernias with potential loss of domain. Subsequent abdominal wall reconstruction techniques are challenging and have led to disappointing short- and long-term results as well as to significant complication rates. Because of the physical disability resulting from the large hernias, for instance, afflicted individuals are often unable to carry on their duties, especially those entailing manual labor.
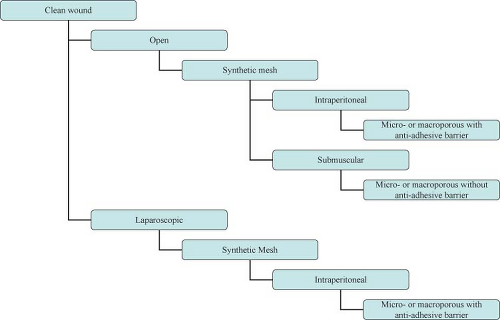
Overall no consensus on abdominal wall reconstruction exists with regard to preferred technique (open vs. laparoscopic), position of mesh (onlay, sublay, intraperitoneal), or type of reinforcement material (synthetic vs. biologic, permanent vs. absorbable, large vs. small pore). More unanimous is the agreement on the need for the use of mesh for the repair of abdominal wall defects resulting from previous incisions, especially if recurrent.
Wide acceptance of prosthetic mesh for the tension-free repair of ventral hernias—especially when used in contaminated fields—presents potential for increased incidence of mesh infections. Treatment of infected meshes and the repair of hernias in infected fields pose significant challenges to the surgeon. In general, the risk of wound infection following synthetic ventral hernia repair varies from 4% to 16%. A prior history of wound infection predisposes the patient to subsequent wound infection in 40% of cases. The fact that wound and mesh infections are risk factors for recurrence creates a vicious cycle potentially resulting in multiply recurrent complex hernias.
The management of wound infections following hernia repair often requires removal of the prosthetic material, leaving a greater defect within an infected field. The treatment alternatives historically have included staged repair with absorbable mesh (polyglycolic acid, i.e., Dexon or polyglactin 910, i.e., Vicryl) or primary repair with myofascial mobilization. Both techniques, unfortunately, are characterized by a high incidence of
recurrence (75% for the former and as high as 52% for the latter). Direct contact of absorbable synthetic meshes with the viscera may also result in adhesions and fistula formation. Recently, a newer generation of synthetic absorbable meshes (e.g., Bio-A®) has been introduced. Although limited literature is available on these new products, initial reports are promising. The new synthetic meshes offer definitive superiority in terms of cost compared to the biologic meshes, so they can be considered as an option.
recurrence (75% for the former and as high as 52% for the latter). Direct contact of absorbable synthetic meshes with the viscera may also result in adhesions and fistula formation. Recently, a newer generation of synthetic absorbable meshes (e.g., Bio-A®) has been introduced. Although limited literature is available on these new products, initial reports are promising. The new synthetic meshes offer definitive superiority in terms of cost compared to the biologic meshes, so they can be considered as an option.
Table 27.1 Synopsis of the Most Commonly Utilized Biologic Meshes | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
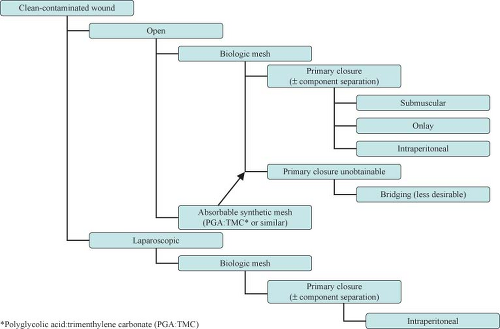
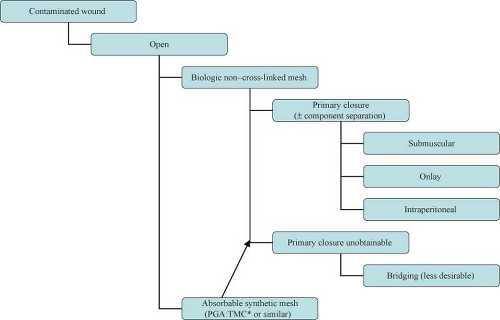
The introduction of biologic grafts has opened a new chapter in the management of complex, contaminated abdominal wall defects. The concept behind these grafts—despite their variance in origins as well as in composition and mechanical properties—is similar: They provide an extracellular matrix scaffold that allows the host cells to start the remodeling process that will result in the laying down of mature collagen, indistinguishable from the native tissue (Table 27.1). The temporary nature of these biomaterials in addition to their ability to promote neo-vascularization, allow them to be implanted in both clean-contaminated and (at least) theoretically contaminated fields. Use in heavily contaminated fields affects the tensile strength of these products and thus leads to higher chance of recurrence. The slower and incomplete remodeling processes of the cross-linked variants of these biologic meshes can further lead to foreign body reactions and chronically draining wounds. Cross-linking is a chemical process (not unlike the tanning of leather) which adds more stability to the extracellular matrix and thus provides graft resistance to the in situ degradation caused by the host collagenase enzymes abundant in contaminated fields. Lack of enzymatic degradation, unfortunately, also adversely affects the neo-vascularization and remodeling process, promoting graft encapsulation and foreign body reaction.
On average, biologic grafts are up to ten times more expensive than their synthetic counterparts. The issue of cost containment where safely feasible in the OR can no longer be ignored by surgeons. The durability of hernia repairs in contaminated fields with biologic grafts has not been adequately prospectively studied. As well the definition of “failures and recurrences” is not always well described in the literature, and some of the thinning and “ballooning” effects seen following biologic graft repair are often not reported as recurrences.
The choice between synthetic and biologic mesh is then dictated by the technique chosen (laparoscopic vs. open), the familiarity of the surgeon with the products, and the cost and the potential for complications, in particular infection.
As previously mentioned, controversies exist in regard to the technique of hernia repair, the need for mesh reinforcement, and the appropriate material to use. One effort in standardizing the approach to complex hernia has been undertaken by the ventral hernia working group (VHWG), a recently established and—though funded by a biologic
mesh manufacturer—credible group. They have sought to evaluate new technologies in hernia repair and to stratify the patients on the basis of their risk of surgical-site occurrence (SSO), particularly surgical-site infection.
mesh manufacturer—credible group. They have sought to evaluate new technologies in hernia repair and to stratify the patients on the basis of their risk of surgical-site occurrence (SSO), particularly surgical-site infection.
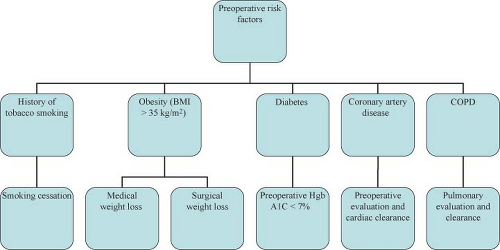
Approach to the complex hernia requires use of preoperative risk assessment algorithms that take into account both patient and hernia characteristics (Table 27.2; Algorithms 27.1–27.5). A careful history should include not only the potential patient co-morbidities but also an accurate review of previous abdominal operations with particular emphasis on hernia repairs and a history of prior wound or mesh infections. Review of previous operative reports, whenever possible, can provide information about the type and location of the implanted mesh. In general, the presence of a previously placed mesh in an overlay position decreases the chance of dense visceral adhesions compared with an intraabdominally placed mesh.
Physical examination should focus on location of the defect (midline vs. eccentric), proximity to bony confinements that might limit mesh overlap (subxiphoid, suprapubic, flank), presence of skin graft or granulation tissue that might become devitalized once the hernia is reduced, and assessment of potential loss of abdominal domain.
In order to minimize potential complications, certain patient risk factors can be optimized prior to surgery (Table 27.2).
Preoperative imaging studies are helpful for defining the anatomy, especially in the setting of multiple previous repairs. Although CT scan is considered the gold standard preoperative test, MRI can be helpful in differentiating “ballooning” or pseudo-recurrence from true recurrence after previous biologic mesh repair. Ultrasonography is rarely used as a preoperative screening tool for complex hernias.
Contaminated or Potentially Contaminated Operative Fields
Clinical scenarios that may lead to an infected or potentially contaminated hernia field include early abdominal wall reconstruction after open abdomen damage control procedures, incarcerated hernias, infected mesh, enteric exposure, presence of ostomies or fistulae.
The main decision points when approaching these complex problems include:
Operative strategy (one-stage vs. multi-stage)
Operative approach (laparoscopic vs. open)
Type of repair (primary vs. mesh)
Type of mesh (synthetic vs. biologic)
Location of mesh (onlay, sublay, interposition)
Table 27.2 Risk Factors for Complication of Hernia Repair | ||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
Operative Strategy (one-stage vs. multi-stage)
Temporary abdominal wall closure is common in the trauma setting when re-exploration is deemed necessary and when the clinical condition warrants expeditious return to the intensive care unit for continuing resuscitation. Evolution in techniques and materials has contributed to use of the temporary abdominal wall closure in the non-trauma setting as well, especially in highly contaminated surgical fields. Once the decision to not close the abdomen is made, the patient is almost guaranteed a ventral hernia. The different absorbable meshes used to close difficult abdomens—in particular polyglycolic acid and polyglactin 910—have resulted in ventral hernias in up to 52% of cases, and adhesions and fistula formation have been described in spite of the absorbable nature of these mesh products. Perhaps the newer absorbable synthetic meshes will provide better short- and long-term results with significant advantage compared with the biologic meshes, although more data is necessary.
The advent of biologic meshes was initially seen as a potential solution to the dilemma of open abdomen/infected field management. It soon became clear, however, that these products have their limitations. First and foremost, they cannot be used to bridge a gap or defect. If so used, hernia recurrences or marked postoperative bulging (pseudo-recurrence) will occur. This is particularly true for products of dermal origin containing a significant elastin component. In addition, these products—when used in infected fields—demonstrate high rates of acute mechanical failure, mesh disintegration, and poor mesh integration (73%, 92%, and 70%, respectively). An alternative one-stage approach with acceptable complication rates in this situation would be component separation and primary fascial closure.
Operative Approach (laparoscopic vs. open)
The choice between laparoscopic and open approach depends on the clinical scenario (Algorithm 27.1–27.5) and the surgeon’s experience and comfort level. Clear advantages of the laparoscopic approach include lower wound-related complication rates, smaller incisions, and shorter hospital stay. Clear disadvantages of the laparoscopic approach, by contrast, include the inability to effectively manage the skin redundancy and hernia sac and the lack of restoration of functional abdominal wall unity. Recently, laparoscopic techniques have been described to obtain primary closure of the fascia in an effort to recreate the functional results obtained with the open repair. Elaboration of these techniques, however, is beyond the scope of this chapter. In general, in the presence of skin grafts and/or infected fields and the loss of abdominal domain, the open approach is preferable. The choice of mesh might also dictate the approach. In fact, if a biologic mesh as reinforcement is chosen, an open repair is usually necessary to obtain primary closure of the defect. Hybrid approaches have also been described in case series, where the main role of laparoscopy is in placing the mesh under adequate stretch and overlap after the hernia defect has been primarily repaired in an open fashion.
Type of Repair (primary vs. mesh)
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree