CHAPTER 7
Immunosuppressive agents
Introduction
Immunosuppressive agents or immunomodulators are general terms referring to drugs used to modulate and inhibit the activity of the immune system. The three main categories of immunomodulators used for the treatment of gastrointestinal diseases are thiopurines, methotrexate and calcineurin inhibitors.
Initially intended mainly for transplant medicine, immunomodulators have proven efficacy in numerous chronic inflammatory conditions and their use in inflammatory bowel disease, while never formally approved, is widely endorsed by professional organizations and have become a cornerstone of the treatment algorithms.
The main uses of immunomodulators in inflammatory bowel disease are:
- induction of clinical remission (methotrexate, cyclosporine (CsA));
- prevention of inflammatory relapses (thiopurines, methotrexate);
- support of tapering down corticosteroids (steroid-sparing effect, thiopurines, methotrexate); and
- prevention of immune neutralization of biological treatment (thiopurines, methotrexate).
In addition, thiopurines and cyclosporine are effective in the treatment of autoimmune hepatitis.
Thiopurines
Introduction of drug class
Azathioprine (AZA) and the closely related 6-mercaptopurine (6-MP) and 6-thioguanine (6-TG) are immunosuppressive drugs that belong to the chemical class of purine analogs. These drugs have proven efficacy and are extensively used for maintenance of remission in Crohn’s disease and ulcerative colitis; approximately four patients would need to be treated to prevent relapse in one. They are used for the prevention of postoperative recurrence in Crohn’s patients. Their co-administration with biological therapy and steroid sparing represent other recommended off-label applications
Basic pharmacology
Mechanisms of action
Thiopurine metabolites are structurally similar to purine nucleic acids. Intermediate metabolites enter purine enzymatic pathways, compete with purines and interfere with the synthesis of purine nucleotides. By incorporation into DNA, the thiopurine end-metabolite 6-thioguanine nucleotide (6-TGN) induces apoptosis of actively proliferating immune cells. While the effect on clonal expansion of lymphocytes is not sufficient to terminate the acute inflammatory process, depletion of antigen-specific memory T-cells effectively prevents further relapses of inflammation
Bioavailability
AZA is well-absorbed, but the bioavailability of 6MP is relatively limited (∼50%).
Metabolism (Figure 7.1)
AZA is quickly and nonenzymatically cleaved to 6-MP.
Figure 7.1 Metabolism of thiopurines. 6-MMP: 6-methylmercaptopurine; 6-MP, 6-mercaptopurine; 6-TGN: thioguanine nucleotide; 6-TU, thiouric acid; AZA, azathioprine; TPMT, thiopurine methyltransferase; XO: xanthine oxidase.
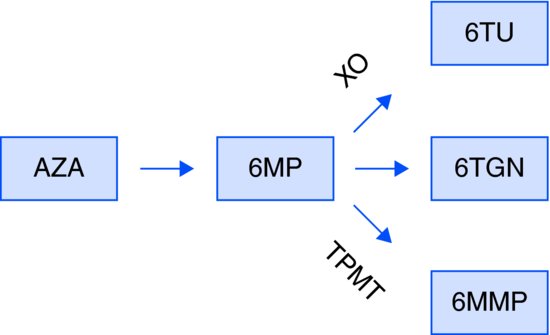
Further metabolism occurs by three competing pathways:
Genetic polymorphism in the TPMT gene makes TPMT activity highly variable
Approximately 0.3% of the population possesses a pair of nonfunctional alleles. Resulting absent TPMT activity renders such patients, if commenced on thiopurines, susceptible to severe and life-threatening myelotoxicity. Another 11% of individuals inherit one functional and one nonfunctional allele (heterozygous), conferring intermediate TPMT activity. The remaining 89% are homozygous for the allele conferring normal activity. Pre-treatment assessment of TPMT status (by geno- or phenotyping) is generally advised and can help to identify patients at risk for severe myelosuppression.
Metabolite monitoring
Assessment of metabolite levels can help to predict the likelihood of both the clinical efficacy and the myelo- and hepatotoxicity (Table 7.1).
Table 7.1 Assessment of metabolite levels
| 6-Thioguanine [6-TG] | > 230 pmol/8 × 108 RBC | Clinical efficacy |
| 6-Methylmercaptopurine [6-MMP] | > 5700 pmol/8 × 108 RBC | Hepatotoxicity |
Special safety concerns
The antigout compound allopurinol effectively blocks xanthine oxidase (XO) purine degradation pathway, shifting thiopurine metabolite flow into the unaffected methylation and 6-TGN-producing trails. The methylation pathway eventually becomes saturated, rending most of the thiopurine into the 6-TGN-producing trail and resulting in rapid and severe myelosuppression. In general, the two drugs should not be administered together.
Occasionally, exaggerated methylation activity (“rapid methylators”) results in the accumulation of hepatotoxic 6-MMP and does not permit adequate dosing of thiopurines. In selected cases, allocation of the metabolite flow to 6-TGN by cautious co-administration of allopurinol enables substantial reduction of the required AZA/6MP dose, indirectly helping to restore tolerable levels of 6-MMP. Such concomitant treatment requires meticulous monitoring of 6-TG, however safety concerns remain.
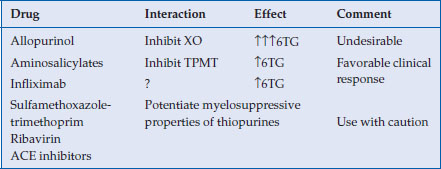
Main drug interaction
Drugs that interfere with the metabolism of thiopurines and potentially myelosuppressive agents should be used with caution (Table 7.2).
Table 7.2 Drugs that interfere with the metabolism of thiopurines and potentially myelosuppressive agents
Adverse effects
Tolerability issues are not infrequent and may limit treatment in up to 20% of the patients.
Adverse effects can be divided into three major categories:
- gastrointestinal intolerance;
- idiosyncratic (including allergic) reactions;
- pharmacologically explainable dose-dependent effects.
The potential risk of infection and neoplastic complications represent additional concerns of chronic immunosuppression.
Gastrointestinal intolerance
Nausea and vomiting are reported by up to 10% of the patients, most often during the first weeks of the treatment, and comprise the major obstacle to patient adherence. These symptoms can be minimized by gradually increasing the dose and administration with meals. Split-dose administration may represent an alternative option. Substituting 6-MP for AZA may also be helpful in some. Blood work to exclude possibility of allergic pancreatitis is mandatory.
Idiosyncratic reactions and hypersensitivity
Fever, rashes, muscle and joint pains are reported in up to 5% of patients and usually represent hypersensitivity to the imidazolyl (rather than mercaptopurine) moiety of AZA. These symptoms may not necessarily recur upon re-challenge with 6-MP.
In contrast, allergic pancreatitis (1%) results from hypersensitivity to the mercaptopurine portion of the AZA molecule and practically precludes switching to 6-MP. Treatment with 6-thioguanine can be considered as an alternative for those who are allergic to AZA/6-MP. Specific side effects such as nodular regenerative hyperplasia limit extensive use of this medication.
Dose-dependent effects
Myelotoxicity
The most significant toxic effect of thiopurines is myelosuppression, which should be anticipated in up to 2% of the patients. If this adverse effect occurs early in treatment, myelosuppression might indicate nonfunctional alleles of TPMT. Delayed myelosuppression is usually related to unforeseen drug interactions or temporary immunosuppression by intercurrent viral infections
Careful monitoring of blood counts is mandatory. Pre-treatment TPMT testing cannot substitute for regular blood work (Table 7.3).
Table 7.3 Monitoring of blood counts
| Required frequency of CBC test | |
| First 4 weeks (or after dose escalation) | On a weekly basis |
| 2nd and 3rd months | Bi-weekly |
| 4th−6th months | Monthly |
| Then (on stable dose) | Every 3 months |
Evidence of modest myelosuppression (WBC < 4 × 109/L or platelet < 120 × 109/L) warrants dose adjustment; profound drop of the counts prompts temporary discontinuation of the treatment.
Macrocytosis
Elevation of mean corpuscular volume (MCV) of red blood cells is frequently observed and, after other possible causes excluded, can actually indicate compliance with the treatment.
Elevated liver enzymes
Methylation is a main metabolic pathway of thiopurines during the first weeks of the treatment and leads to temporary elevation of hepatotoxic 6-MMP levels. Mild and transient elevation of liver transaminases is frequently (13%) observed and does not necessarily signal a chronic, serious liver problem. Temporary adjustment of the dose to allow adequate time for induction of oxidation metabolic pathways is usually sufficient. In contrast, a rapid and steep (X4) elevation of GGT might indicate clinically significant hepatotoxicity and warrants prompt discontinuation of the treatment. Serious problems such as mercaptopurine moiety-related cholestasis, veno-occlusive disease and nodular regenerative hyperplasia are extremely rare.
Infection
Infections are a constant concern for patients receiving chronic immunosuppression. With this concern in mind, it should be noted that treatment of IBD patients with thiopurines has not been associated with the risk of serious infection. There is also no evidence for a diminished immune response to vaccination.
Association with neoplasia
Lymphoproliferative disorders
Chronic immunosuppression results in defective T-cell immunosurveillance, raising a concern of potential development of lymphoproliferative disorders. Thiopurines have been reported to increase the risk of lymphoma up to five-fold, yet the absolute risk appears to be quite low.
Hepatosplenic T-cell lymphoma (HSTCL)
HSTCL is an extremely rare and very aggressive lymphoproliferative disorder reported in IBD patients treated with thiopurines, with or without concomitant treatment with a TNFα antagonist.
Nonmelanoma skin cancer
The use of thiopurines is associated with an increased risk of nonmelanoma skin cancer in IBD patients. Periodic examination by a dermatologist and appropriate sun protection are advised.
Dosing information (Table 7.4)
The optimal protocol for introducing treatment with thiopurines has not been established. Most experts advocate a low starting dose, with subsequent titration of the dose according to clinical response and adverse events; some use decreased daily doses in TPMT heterozygotes.
Table 7.4 Dosing information
| Drug | Initial dose |







