Epidemiology
Race/ethnicity:
Whites and Blacks > Hispanic, Native American, Asian Jews > Non-Jews
Geography:
Northern climates > Southern Scandinavia, North America, Europe > Asia, Africa, South America, Japan, Spain
Gender:
CD: Female > Male
UC: Male > Female
Age:
CD: Third decade
UC: Fourth decade
Residence:
Urban > Rural
Indoor > Outdoor
Risk factors
Diet:
Sugar consumption – ↑ CD
ETOH – ↓ UC
Margarine – no association
Coffee – no association
Fiber – no association
Food additives – no association
Childhood diarrheal illness ↑ IBD
Higher socioeconomical status ↑ IBD
Oral contraceptive use ↑ IBD
Cigarettes – ↑ CD
↓ UC
Appendectomy ↓ ulcerative colitis
NSAIDS – ↑ symptoms of IBD
The prevalence of IBD (incidence x disease duration) greatly varies throughout the world.
IBD is found in the more temperate climates of North America and Europe. Studies from these regions show prevalence rates much higher than those in Asia, South America, or Africa.
It is generally recognized that both CD and UC have been increasing in incidence suggesting an environmental effect, since a genetic factor would probably not influence disease rates so rapidly.
CD most commonly occurs in the third decade of life, while UC is more common in the fourth decade.
There may be a bimodal distribution of disease incidence with a second peak in the sixth or seventh decade
Recent case–control studies in USA suggest a similar incidence in blacks and whites.
The incidence is consistently higher in Jews than in the non-Jewish population in most countries studied.
IBD is more common in urban, “indoor” populations of individuals of middle to upper socioeconomic status, suggesting the “hygiene” hypothesis that relates the lack of early exposure to environmental antigens to the later development of disease.
There is very little evidence that a specific dietary factor causes disease.
Childhood diarrheal illness and oral contraceptive and nonsteroidal anti-inflammatory drugs (NSAIDs) use are measurable risk factors for IBD.
Smoking increases risk of developing Crohn’s de novo and increases risk of recurrence after surgical resection.
Conversely, smoking is protective for UC, as is prior appendectomy.
Genetic Disease Determinants in IBD
Approximately 20 % of patients with IBD will have a family member also afflicted.
Genome-wide association studies (GWAS) have identified areas (loci) in the human genome that are associated with the various forms of IBD.
As of mid-2009, an excess of 32 loci (most containing several potential disease-causing genes) was found for CD alone.
UC appears to have less of a genetic component to its pathobiology but similarly has at least 15 loci associated with it (Fig. 27.1).
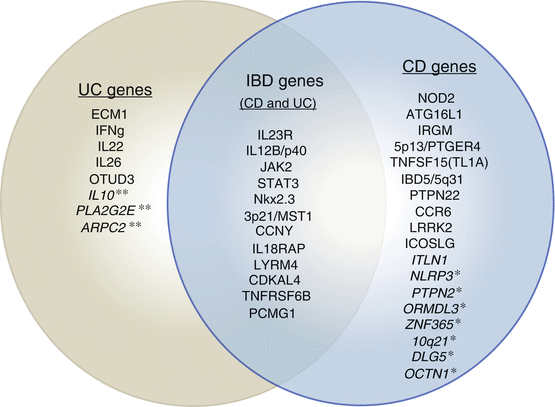
Fig. 27.1
Genes implicated in the pathogenesis of Crohn’s disease and ulcerative colitis (as of late 2009). Some genes seem to play a role in both diseases. Not all genes have been confirmed by second investigators. * CD genes not fully tested in UC. ** UC genes not fully tested in CD
IBD may be the consequence of an altered host immune response to various environmental factors, most probably enteric or commensal bacteria within the gut.
Other factors may also play a role, such as smoking or NSAID use that further alters the host immune system or affects its function (Fig. 27.2).
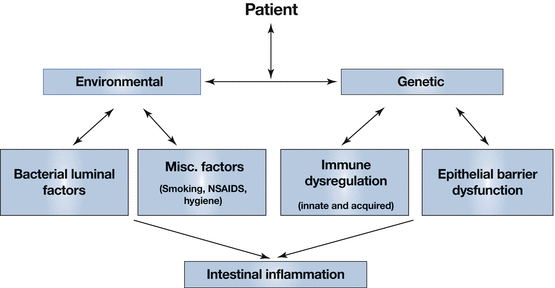
Fig. 27.2
Pathogenesis of inflammatory bowel disease incorporating a genetic (predisposition) component and an environmental aspect, each of which may play a varying role in the individual patient
The first CD-associated genetic mutation was found in the NOD2/CARD 15 gene on chromosome 16.
This gene’s protein product recognizes muramyl dipeptide, a component of bacterial cell walls, and activates NF-kappa B, a potent second messenger involved in immune regulatory mechanisms.
Though a homozygous (double) mutation in this gene increases the risk of CD approximately 30-fold, any one patient with a mutation will still only have an approximately 2.5 % risk of developing CD.
Autophagy, i.e., the cell’s ability to destroy and recycle defective cytoplasmic molecules including invading bacteria, is compromised by mutations in the ATG16L1 and IRGM genes, which have been associated with CD.
Mutations in the acquired immune system, especially those involving the IL-23 and IL-12 signaling pathways, have been found.
Various alleles in other molecules found in the IL-23 pathway including JAK2, STAT3, and IL12B have also been linked with CD and UC.
Experimentally, blockade of the IL-23 pathway may compromise bacterial clearance in some models, reinforcing the concept that IBD is the consequence of an abnormal immune response to commensal gut bacteria.
IBD-associated mutations have been found in many other genes, including DLG5 (coding for an epithelial cytoplasmic scaffolding protein), NKX2-3 (a developmental protein found in the lymphoid tissue of gut and involved in the expression of lymphocyte trafficking molecules), PTPN2 (involved in T-cell-dependant B-cell function), and IL-10R (receptor).
Signs and Symptoms
Crohn’s Disease
CD can affect any portion of the gastrointestinal (GI) tract from the mouth to the anus and it is usually discontinuous.
The inflammation of CD involves the entire bowel wall.
The most common complaints of any patient with CD are abdominal pain and diarrhea, being found in over 75 % of patients.
Weight loss, fever, and bleeding are present in approximately 40–60 % of patients.
Anal symptoms of abscess and/or fistula occur in 10–20 % of patients.
CD is most frequently found in the ileocecal region (approximately 40 %).
Colonic disease is found in approximately 30 % and correlates with diarrhea and bleeding.
30 % have disease confined to the small bowel proximal to the terminal ileum and correlates with abdominal pain and bloating.
Anal disease is associated with terminal ileal and colonic distributions of disease.
Vienna classification of CD segregates patients into three categories based on behavior: inflammatory (B1), stricturing (B2), and fistulizing (B3).
Patients will frequently change categories as disease progresses.
Approximately 50 % of CD patients will be in clinical remission.
The majority of patients (60–75 %) will have alternating years of quiescence and disease activity.
10–20 % will have either a chronic, unremitting course or repetitive annual flaring of disease.
The inflammation of UC characteristically starts in the rectum and extends proximally.
If small bowel is involved, CD should be suspected.
Thus rectal disease results in increased stool frequency, hematochezia, and tenesmus. Diarrhea is a frequent symptom.
Constipation with a sense of incomplete evacuation can be a complaint in 20–25 % of patients.
With increasing severity and extent of disease, nausea, vomiting, and weight loss ensue.
Toxic megacolon is a moniker that should be discarded, since severe life-threatening colitis may occur without colonic dilatation.
Extraintestinal Manifestations
The most common non-GI complaints in IBD patients relate to the musculoskeletal system.
Osteopenia and osteoporosis are very common, in part due to therapeutic steroid use, occurring in as many as 50 and 15 %.
There is a 40 % increased risk of bone fractures in IBD patients.
Peripheral arthritis usually affects multiple small joints and has little relation to gastrointestinal disease activity.
Axial arthritis (ankylosing spondylitis) is associated with certain HLA subtypes (B27) and is found in approximately 5 % of both CD and UC patients. Its severity commonly parallels disease activity.
Recently, anti-tumor necrosis factor (TNF) therapies have been shown to be effective in both CD and the arthropathy of IBD.
Pyoderma gangrenosum and erythema nodosum occur in approximately 0.5–5 %.
These and oral lesions such as aphthous stomatitis and pyostomatitis vegetans are more commonly associated with CD than UC and commonly parallel underlying gastrointestinal disease activity.
There is a reported increased rate of psoriasis and eczema in IBD patients that does not parallel disease activity.
Primary sclerosing cholangitis (PSC) has a reported incidence of approximately 3 % in both CD and UC patients.
It may present independent of intestinal disease activity.
Colectomy in UC patients does not affect the progression of liver disease.
PSC in the UC patient increases the risk for malignant disease in both the colon and the hepatobiliary system.
Iritis, uveitis, and episcleritis can affect 2–8 % of patients with UC and CD, respectively, and are generally unrelated to disease activity.
Iritis and uveitis present as blurred vision, eye pain, and photophobia. These require prompt treatment to avoid scarring and even blindness.
There is an identified increased risk of deep venous thrombosis (DVT), mesenteric thrombosis, and pulmonary embolism (PE).
Decreased protein S and antithrombin III levels due to mucosal loss and increased levels of acute-phase reactants including factors V and VIII have been implicated.
Disease Severity Assessment
The CD activity index (CDAI) is the most commonly used method for quantification of disease severity in CD (Table 27.2).
Table 27.2
The Crohn’s disease activity index (CDAI)
Item calculation
Data-collected weighing factor
No. of liquid stools
7-day diary
Sum of 7 days
2
Abdominal pain
0–3 scale, 7-day diary
Sum of score for each day
5
General well-being
0–4 scale, 7-day diary
Sum of score for each day
7
Symptomsa
At clinic visit
Sum (6 total) possible
20
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree

 Get Clinical Tree app for offline access
Get Clinical Tree app for offline access




