Chapter 68 Hydatid disease of the liver
Overview
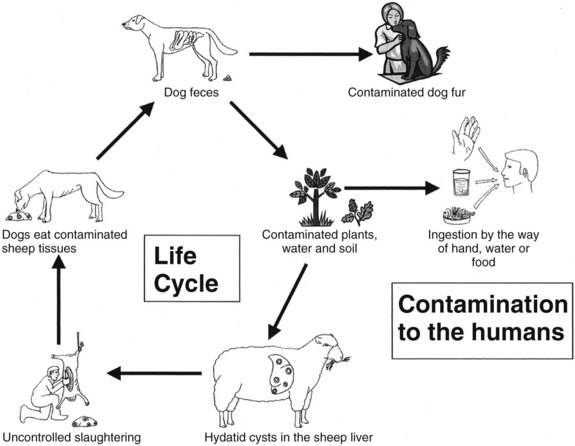
Hydatid disease, or echinococcosis, is a widespread zoonotic parasitic disease caused by a tapeworm that continues to be a clinical and public health problem worldwide, especially in areas where animal husbandry and subsistence farming form an integral part of community life. Hydatidosis infects a large number of wild and domestic animals and humans, and the larval stage of the disease develops into a hydatid cyst. Hydatid disease is most frequently caused by Echinococcus granulosus, and the liver is the most commonly involved organ in two thirds of patients, although it may affect any part of the body, either as a primary or secondary event (Shaw et al, 2006). The life cycle of Echinococcus requires a definitive host, which is often a dog, and an intermediate host, which is commonly sheep. Humans become accidental intermediate hosts when they become infected after ingesting ova passed in dog feces.
Pathogenesis and Etiology
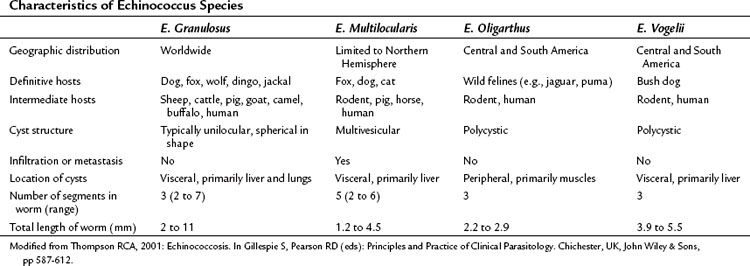
Although 16 species and 13 subspecies were originally described in the genus Echinococcus, molecular epidemiologic studies have led to the recognition of only four clinically important species: E. granulosus, E. multilocularis (E. alveolaris), E. oligarthrus, and E. vogeli (Thompson, 2001). Recently, a new strain, E. shiquicus, has been identified on the Tibetan plateau, but to date no human infection has been described (Shaw et al, 2006). The characteristics of the four Echinococcus species are summarized in Table 68.1. E. granulosus is the most common and is the main focus of this chapter. E. multilocularis, a rare and aggressive form of hydatid disease, is discussed separately at the end of the chapter.
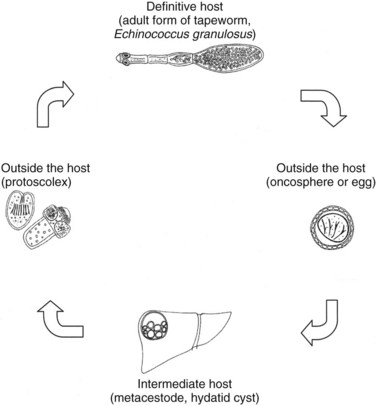
E. granulosus is a small, hermaphroditic tapeworm about 5 mm in length. The tapeworm consists of a head, or scolex, and a body, or strobila, with three or four proglottids (Fig. 68.1). The last one is the largest and bears the mature eggs. The eggs contain a hexacanth embryo that has three pairs of lancet-shaped hooklets. The life cycle of E. granulosus requires two hosts, a carnivore and an herbivore (Fig. 68.2). The adult tapeworm lives in the intestine of the dog, which is the most common definitive host for E. granulosus. Worms release large numbers of infected eggs that pass out in the dog feces and contaminate soil, water, and plants. The eggs are ingested by the intermediate host (humans are accidental intermediate hosts), the eggs hatch, and the embryo migrates through the intestinal wall into the portal system. Most embryos lodge in the liver, mainly in the right lobe because of preferential portal flow; there they develop into hydatid cysts within months to years. Embryos may escape this first filter and lodge in the capillaries of the lung, where they develop. A small percentage of embryos find their way into the systemic circulation and involve other organs, including the spleen, kidney, brain, bone, or any other site. Most patients have single organ involvement. In the liver, the parasite develops into the larval stage, the hydatid cyst, which is filled with fluid and contains hundreds of protoscolices. For the life cycle to be complete, a canine host must ingest the hydatid cyst or its contents, which commonly occurs when infected sheep are slaughtered and organs containing hydatid cysts are fed to dogs (Krige & Beckingham, 2001).
Epidemiology
The disease occurs principally in sheep-grazing areas, especially where dogs are allowed to stray and eat uncooked viscera. Echinococcosis is endemic in many Mediterranean countries, the Middle and Far East, South America, and South and East Africa. The incidence of disease in humans in endemic areas depends on the level of health care and veterinary control. The incidence of human hydatidosis is often established by the number of surgically treated patients. The yearly incidence of human hydatidosis per 100,000 population ranges from 0.4 in Switzerland and Wales to 196 in Turkana in the northwest of Kenya. This high incidence is due to the close relationship the Turkana people have with their dogs: they sleep with them for warmth in the desert nights, and dogs are kept as “nurses” to lick babies clean after they vomit or defecate (Richards, 1992; Watson-Jones & Macpherson, 1988). This domestic intimacy results in many of the population becoming infected when very young (Morris, 1992).
As a result of slow growth, cysts usually become symptomatic a few years after infection, in adolescence or early adulthood. Infected adults may become symptomatic later in life. Host immunity may overcome infection, resulting in a nonviable echinococcal cyst without the person ever becoming symptomatic. Humans are accidental hosts and play little part in the transmission of the disease, making them so-called dead-end hosts, and the disease is not transmitted from human to human (Shaw et al, 2006).
Development of a Hydatid Cyst
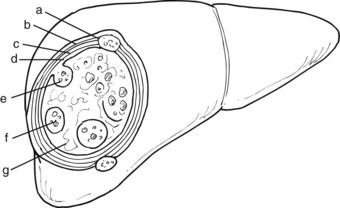
When the parasite reaches the liver parenchyma, it develops into a cystic larval phase, which is visible within 3 weeks and may measure up to 3 cm in diameter after 3 months. The mature E. granulosus cyst consists of three layers: a germinal layer, a laminated layer, and an ectocyst. The inner, germinal layer surrounds the fluid-filled central hydatid cavity and in turn is surrounded by the laminated layer. These two layers together form the endocyst. Compression of the host tissue around the endocyst produces a fibrous layer known as the ectocyst or pericyst (Figure 68.3).
The germinal layer, also called the germinative membrane, is the living component of the parasite. Undifferentiated cells in the germinal layer produce invaginations into the cyst cavity, forming brood capsules that contain protoscolices, which are released into the cyst fluid. The germinal membrane secretes fluid into the cyst and is the source of daughter cysts (Fig. 68.4). The presence of daughter cysts creates multivesicular cysts, which are more common in adults than in children. Daughter cysts have a structure similar to the mother cysts, including a laminated and germinative membrane, cyst fluid, brood capsules, and protoscolices. The only difference is the absence of an adventitial layer (Krige & Beckingham, 2001).
The ectocyst or pericyst is a fibrous capsule that develops from host tissue as an inflammatory reaction to E. granulosus. This thick fibrous layer is present in hydatid cysts in the liver and spleen but is absent in pulmonary and brain hydatid cysts. Vascular structures and bile ducts in the adventitial layer remain intact and patent despite enlargement of the cyst and may result in postoperative bleeding or bile leaks after partial pericystic resection. The blood supply of the adventitial layer is abundant and results in the appearance of a hypervascular rim or halo around the cystic cavity on computed tomography (CT) scans after contrast injection. No clear cleavage planes are apparent between the adventitial layer and the surrounding normal host tissue, and the cyst is not readily separable from the surrounding parenchyma. With time, the adventitial layer may calcify, either partially or totally (Krige & Beckingham, 2001).
An uncomplicated hydatid cyst typically contains a clear, colorless, odorless fluid secreted by the germinal membrane. Sodium, chloride, and bicarbonate concentrations are the same in the fluid as in the patient’s plasma, whereas potassium and calcium levels are lower. In uncomplicated cysts, hydatid fluid is sterile. Bile-stained cyst fluid indicates a cystobiliary communication. When superadded infection is present, the cyst fluid appears frankly purulent; in degenerated cysts, the fluid becomes turbid. Spillage of hydatid fluid content as a result of traumatic or iatrogenic rupture produces implantation of protoscolices and secondary cysts on surrounding viscera, known as secondary hydatidosis (Krige & Beckingham, 2001). Although any segment of the liver can be involved, the location of liver hydatid cysts seems to be related to the respective volume of each lobe of the liver; thus a higher involvement of the right lobe is observed, especially in segments VII and VIII (Kayaalp et al, 2003).
Complications
Compression
As the cyst grows, enlargement tends to occur toward the surface of the liver and Glisson’s capsule, compressing the surrounding parenchyma and leading to compensatory hypertrophy of the remaining liver tissue. Sometimes an entire liver lobe can be replaced by a large cyst without any symptoms. Depending on the location, large cysts can cause compression of the adjacent bile ducts, portal or hepatic veins, or vena cava that causes obstructive jaundice, portal hypertension, or Budd-Chiari syndrome (Moreno-Gonzalez et al, 1994).
Rupture into the Biliary Tract
Intrabiliary rupture is the most common complication of liver hydatid cysts (Iscan & Duren, 1991; Yilmaz & Gokok, 1990). Cystobiliary communications that occur after rupture of a cyst into the bile ducts can be minor or major. Minor communications are usually asymptomatic and are revealed postoperatively by the presence of a bile leak, whereas major communications cause obstructive jaundice and cholangitis (Figure 68.5). In histologic studies of the pericyst wall, numerous biliary ducts of various sizes that communicate with the residual cavity have been demonstrated (Gahukamble et al, 2000), suggesting the existence of biliary communications in most hydatid cysts (Langer et al, 1984). The reported incidence of clinically evident cystobiliary communications rates vary from 2.6% to 28.6% (Langer et al, 1984; Ozmen et al, 1992). In a study by Kayaalp and colleagues (2003), the incidence of cystobiliary communications was 37% and the incidence of clinically apparent biliary leakage was 26%. The incidence of cystobiliary communications depends largely on the criteria used for defining the communications. Morel and colleagues (1988) showed a 36% rate of bile leaks when doing routine intraoperative cholangiography.
Preoperative and intraoperative determination of biliary communication is important. Kayaalp and colleagues (2002) found that the risk of biliary-cyst communication was higher in male patients (40.9% vs. 10.4%; P < .01) and in those with abnormal preoperative serum alkaline phosphatase and γ-glutamyltransferase (GGT); it was also higher in patients with multiple cysts, multilocular (23.8%) and degenerated cysts (24%) compared with unilocular cysts (12.5%); cysts near the biliary bifurcation, and in the presence of bile-stained or purulent cyst contents compared with others (61.9% vs. 2%; P < .001). Although the size of the cyst did not seem to be significant with regard to bile leakage in this study, Atli and colleagues (2001) found that a cyst diameter greater than 10 cm was an independent clinical predictor for the presence of intrabiliary rupture.
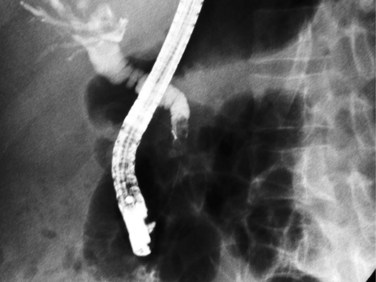
A major biliary communication has been defined as a fistula greater than 5 mm in diameter, a communication between the cyst and the main bile duct, or both (Bourgeon, 1985). The reported incidence of major biliary-cyst communications ranges from 5% to 10% (Zaouche et al, 2001). When large segmental ducts are involved, daughter cysts may enter the bile duct and cause obstructive jaundice or cholangitis or both. Ultrasound (US) and CT scans may show a detached membrane in the cyst cavity associated with dilated intrahepatic and extrahepatic bile ducts (see Fig. 68.5). Endosonography may also detect cystic material in the extrahepatic bile ducts. Endoscopic retrograde cholangiopancreatography (ERCP) is useful to confirm biliary obstruction that results from hydatid material and facilitate treatment with an endoscopic sphincterotomy and extraction of the hydatid debris with a balloon or basket (Fig. 68.6; Ozaslan & Bayraktar, 2002).
Rupture into the Bronchial Tree
A bile leak into large hydatid cysts in segments IVa, VII, and VIII of the liver with secondary infection results in inflammatory adhesions to the diaphragm and the pleurae, which may erode spontaneously into the pleural space, pulmonary parenchyma, and bronchi and lead to a bronchobiliary fistula. This classic presentation is confirmed when the patient reports coughing up “grape skins” (Krige & Beckingham, 2001).
Rupture into the Peritoneum
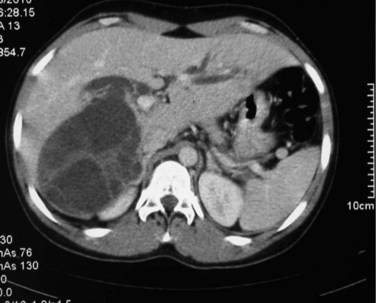
Intraperitoneal rupture of a hydatid cyst is an uncommon clinical presentation, even in endemic regions, with an incidence ranging from 1% to 8% (Sozuer et al, 2002). Rupture can occur spontaneously. Although this complication may be totally silent (Abdel Hameed & Abu Aisha, 1987), abdominal pain, nausea, vomiting, and urticaria are the most common symptoms, and acute abdominal signs—such as guarding, rebound, and tenderness—are generally present. This complication should be included in the differential diagnosis of an acute abdomen in endemic areas. The release of brood cystic fluid into the peritoneal cavity leads to multiple cysts throughout the peritoneal cavity, ultimately resulting in gross abdominal distension, ascites, and intestinal obstruction. US and CT may be helpful in defining the cyst with a detached membrane and intraabdominal fluid in patients with a ruptured hydatid cyst (Fig. 68.7).
Rupture into Other Cavities or Organs
Rupture into the gastrointestinal tract that involves the stomach and the duodenum has been reported (Diez Valladares et al, 1998). Isolated cases of rupture of liver hydatid cysts into the pericardium (Thameur et al, 2001) and into large vessels, including the inferior vena cava, have also been described (Karunajeewa et al, 2002).
Diagnosis
Symptoms
Small (<5 cm in diameter) and uncomplicated cysts usually are asymptomatic and detected incidentally during a radiologic examination of the upper abdomen or right upper quadrant. The expansion of larger cysts or the inflammatory reaction around a cyst causing irritation of the adjacent parietal peritoneum may cause moderate pain in the right upper quadrant or in the lower chest. Acute abdominal pain usually indicates an infected hydatid cyst or rupture into the peritoneal cavity. When antigenic cyst fluid is released into the circulation, especially after rupture into the peritoneal cavity, a variety of acute allergic manifestations may occur, such as urticaria, anaphylactic attacks, or episodes of asthma (Vuitton, 2004). Extrusion of cyst contents into the biliary tree may lead to absorption of the hydatid antigen in sensitized patients, resulting in similar allergic manifestations (Little, 1976). Clinical features of rupture into the biliary tree are recurrent colicky pain and jaundice, with or without resultant fevers and chills, mimicking obstructing bile duct stones. Bronchobilia resulting from a hepatobronchial fistula and ascites resulting from pressure on hepatic veins or inferior vena cava or both (Budd-Chiari syndrome) are rare clinical presentations.
Laboratory Tests
Even large hydatid cysts of the liver may not alter liver function tests, and transaminase levels are usually normal. Cholestatic enzymes, such as alkaline phosphatase and GGT, can be mildly elevated in about one third of patients, especially in patients with biliary compression (Kayaalp et al, 2002). Elevated bilirubin levels (>1 mg/dL) with elevated alkaline phosphatase and GGT levels are highly suggestive of a cystobiliary communication. White blood cell counts are elevated only if the cyst has become secondarily infected. Eosinophilia (>3%) occurs in 25% to 45% of patients with hydatid cysts in Western countries, but this is a nonspecific finding in endemic areas (Pitt et al, 1986). Serum immunoglobulin levels are elevated in 31% of patients with hydatid liver cysts (Kayaalp et al, 2002).
Radiology
Ultrasound and Computed Tomography
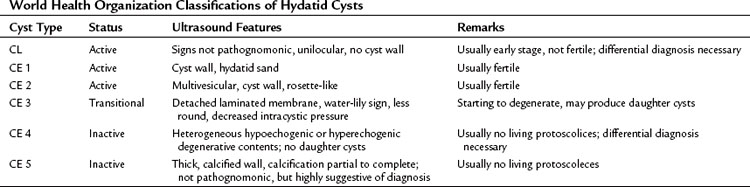
Hydatid cysts appear as well-defined, circumscribed cystic lesions with a clear membrane; they do not infiltrate surrounding liver tissue, and cysts are staged according to the content patterns. Staging is important for using a uniform nomenclature to allow a rational comparison of different management strategies. Although most staging protocols were based on US findings in the past, CT findings can be adapted easily to these systems. The World Health Organization (WHO) Informal Working Group on Echinococcosis (2003) described an ultrasound classification system that intended to follow the natural history of hydatid disease. Based on several studies and classifications, liver hydatid cysts can be divided into six types (Table 68.2; Beggs, 1983; Gharbi et al, 1981; McCorkel & Lewall, 1985):
1 CL type: a well-circumscribed liquid image with a clearly defined wall that is often difficult to differentiate from a simple biliary cyst and corresponds to an early stage of development
2 CE 1 type: a concentric, hyperechogenic halo around the cyst (Fig. 68.8), which may contain free-floating hyperechogenic foci called hydatid sand.
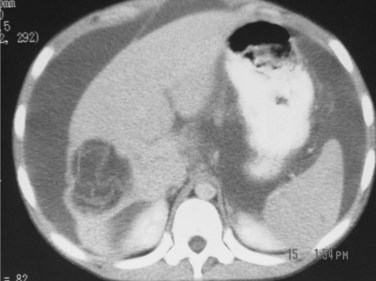
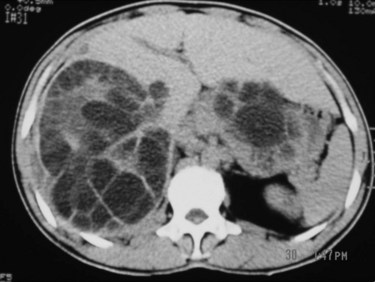
3 CE 2 type: multivesicular cysts that have the most characteristic appearance, with the “daughter” and “granddaughter” cysts identified by honeycomb, rosette, spoked-wheel, or cluster images; can also have some free cyst fluid within the main cavity or may be full of daughter cysts without any free fluid (Fig. 68.9).
4 CE 3 type: partial or total detachment of the laminated layer with floating and undulating hyperechogenic membranes showing the dual wall and “water lily,” and “water snake” signs.
5 CE 4 type: cysts that contain cystic and solid components together without visible daughter cysts.
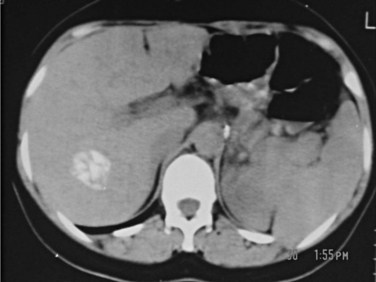
6 CE 5 type: cysts with a matrix or amorphous mass with a solid or semisolid appearance, often a limited amount of calcification in the rim of the host adventitial tissue. This is the least typical of the cysts and may pose a diagnostic problem; they can be mistaken for a tumor, hepatic abscess, or hemangioma. Calcification in the cyst wall and hypoechogenic lacunar structures in the matrix do not indicate that the cyst is dead; a completely calcified cyst (eggshell appearance) is accepted as an effete or dead cyst (Fig. 68.10).
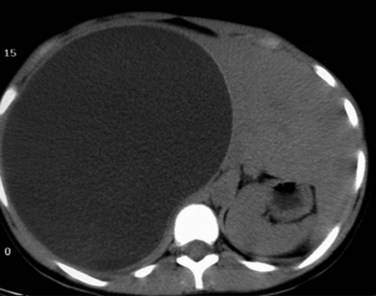
FIGURE 68.8 Computed tomographic scan of a univesicular hydatid cyst shows a single cyst with clear cyst contents.
CL, CE 1, and CE 2 are considered active, fertile cysts. CE 3 is a transitional cyst believed to have begun degeneration. CE 4 is a degenerated cyst and CE 5 is a calcified cyst. The degree of calcification varies from partial to complete. CE 4 and CE 5 are accepted as inactive cysts (Shaw et al, 2006).
Other Imaging Studies
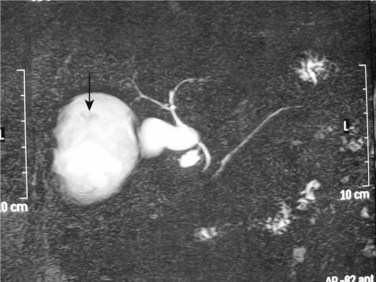
Plain radiography of the abdomen is of limited value in the diagnosis of hydatid liver cysts. A plain radiograph of the chest can reveal concurrent hydatid cysts of the lung. On T2-weighted magnetic resonance imaging (MRI), hydatid liver cysts may have a low signal intensity rim. This is a characteristic sign of hydatid disease that represents the outer, collagen-rich laminated membrane of the cyst. When present, daughter cysts are seen as cystic structures attached to the germinal layer that are hypointense relative to the intracystic fluid on T1-weighted images and hyperintense on T2-weighted images (Pedrosa et al, 2000). MRI is more specific than CT, especially if intracystic fat density is present, which suggests cystobiliary communication (Basaran et al, 2005). In cysts with biliary complications, MR cholangiography can provide good visualization of the intrahepatic and extrahepatic biliary tree and its relationship with the hydatid cyst and cystobiliary communications (Fig. 68.11; Little et al, 2002).
Serology
Immunoelectrophoresis
The diagnostic value of hydatidosis with immunoelectrophoresis ranges from 91% to 94% for hepatic cysts and 69% to 70% for pulmonary cysts (Varela-Diaz et al, 1983). Immunoelectrophoresis is not suitable for epidemiologic surveillance; rather, it is used for posttreatment follow-up.
Enzyme-Linked Immunosorbent Assay
Sensitivities for enzyme-linked immunosorbent assay (ELISA) vary from 64% to 100% depending on the antigens used (Coltorti, 1986; Iacona et al, 1980; Rickard, 1984). ELISA can also be automatized for large-scale epidemiologic studies. Selected test antibodies affect its value on posttreatment follow-up. Immunoglobulin (Ig) G assay may remain positive 4 years after successful treatment, so it is not a suitable test for posttreatment follow-up; IgM assay has been reported to be negative after 6 months of successful treatment (Zhang et al, 2003).
Blotting
Blotting allows molecular weight analysis of the antigens detected by the patient’s serum. Western blotting with purified antigens has proved to be very useful in the diagnosis and postsurgical monitoring of hydatidosis patients (Doiz et al, 2001). The Arc 5 antibody test is a specific precipitation during electrophoresis of blood of hydatid cyst patients, with a specificity of 91%. Purification of antigens strongly affects the diagnostic value of the tests. Purified fractions enriched in antigens 5 and B and in glycoprotein yield a sensitivity of 95% and specificity of 100% (Sbihi et al, 1996).
Treatment Indications and Methods
Three treatment options are currently available for hydatid disease of the liver: surgery, which remains the most efficient treatment and the therapy of choice; percutaneous aspiration; and medical treatment. In general, hydatidosis is a public health problem, especially in developing countries, and the specific treatment selected may depend on social circumstances and the medical expertise available (Shaw et al, 2006).
Since the 1990s, percutaneous treatment has been increasingly used. Surgery has the advantage of removing the parasitic content of the cyst and the cyst wall and dealing with any associated complications. Although surgery may be technically demanding in patients with large and complicated hydatid cysts, advances in liver surgery have made complex operations safer and have reduced morbidity (Krige & Beckingham, 2001).
Conservative Management
Asymptomatic and small (<5 cm) CL-type cysts can be followed up with a wait-and-see policy with serial US examinations (Buttenschoen & Buttenschoen, 2003). Similarly, densely calcified hydatid cysts are accepted as dead cysts and can be monitored without any specific therapy.
Surgical Treatment
The literature on the surgical treatment of hydatid liver disease describes a variety of different techniques, ranging from simple evacuation to major liver resection. These techniques can be divided into two broad groups, conservative and radical. The conservative method involves inactivation of protoscolices and removal of the cyst contents; radical methods include total excision of the cyst and pericyst layers along with a portion of surrounding liver. Although no prospective randomized data compare radical and conservative surgery, most surgeons, especially in endemic areas, prefer conservative surgery, but some experienced liver surgeons may at times use pericystectomy or hepatectomy to deal with liver hydatid cysts. The principles of liver hydatid surgery include inactivation of protoscolices within the cyst fluid, evacuation of the cyst contents and prevention of spillage of the cyst contents, secure closure of any cystobiliary communications, and management of the residual cyst cavity (Terblanche & Krige, 1998).
Prevention of intraoperative spillage of the hydatid cyst contents is an important step during the procedure. Several methods have been described to avoid spillage. First introduced by Saidi and Nazarian (1971) and later by Aarons and Kune (1983)
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree