Hybrid Robotic and Fully Robotic Procedures
Susan M. Cera
Rectal cancer surgery is technically challenging because of the limited confines of the pelvis and the close proximity of the presacral veins, autonomic nerves, and reproductive organs. In 1979, the procedure that is known as total mesorectal excision (TME) was introduced by Dr Heald (1) and is now universally accepted as the gold standard for treatment of rectal cancer. The technique involves precise dissection of the avascular plane between the presacral fascia and the fascia propria of the rectum. The goal for optimal oncologic outcome is total excision of the mesorectum including an intact mesorectal envelope without defects and microscopically tumor-free radial and distal margins. The secondary goal is autonomic nerve preservation relating to quality of life.
Rectal cancer surgery has gone through an evolution of change in the era of minimally invasive surgery. Since the first laparoscopic colectomy in 1991, the use of laparoscopic surgery for colorectal cancer has been increasing. Appropriate oncologic outcomes for colon cancer have been validated in randomized trials studies such as the COST trial (2). Likewise, laparoscopic low anterior resection (LAR) with TME has several advantages when compared with open LAR, including reduced postoperative pain, faster recovery of bowel function, improved quality of life, and decreased hospital stay and disability. However, the laparoscopic approach to LAR has inherent technical limitations, such as a two-dimensional view, limited dexterity of the long, straight instruments, and fixed instrument tips. Consequently, this technique has been proven to have a steep learning curve with a high rate of conversions. The British CLASSIC trial, a large prospective randomized study comparing laparoscopic to open colorectal surgery, reported a 34% conversion rate for laparoscopic approach to rectal resection (3).
The Intuitive Surgical® Da Vinci surgical™ system (Intuitive Surgical®, Sunnyvale, CA), FDA-approved in 2000, was specifically developed to compensate for the technical limitations of the laparoscopic approach. The magnified vision is 10 times that of the human eye and, when the image visualized through the view finder on the surgeon’s console, is three-dimensional. Motion scaling is a feature that translates small hand movements outside the patient’s body into precise movements inside the body. As the movements are transferred from the handle to the tip of the instrument, tremors and small movements are filtered for enhanced dexterity and smoother motion especially
during fine dissections and suturing under microscopic magnification. Motion scaling is designed to allow greater precision than is normally achievable in open and laparoscopic surgery. Finally, the tips of the robotic instruments encompass endowrist technology demonstrating the same full range of motion as a human wrist and can therefore rotate 360 degrees and bend with 90 degrees of angulation.
during fine dissections and suturing under microscopic magnification. Motion scaling is designed to allow greater precision than is normally achievable in open and laparoscopic surgery. Finally, the tips of the robotic instruments encompass endowrist technology demonstrating the same full range of motion as a human wrist and can therefore rotate 360 degrees and bend with 90 degrees of angulation.
Robotic LAR with TME for rectal cancer has been described in two ways. The first is a hybrid of laparoscopy and robotics. Vessel division and mobilization of the splenic flexure are accomplished laparoscopically followed by positioning of the robot between the patient’s legs for the robotic TME. This approach was the mainstay procedure using the original design of the Da Vinci system with three arms. The second robotic approach, developed by Dr Seon-Hahn Kim of Seoul, Korea, involves robotic use for all portions of the procedure including vessel division, mobilization of the splenic flexure, and the TME. Fully robotic LAR has only been possible since the release of the Da Vinci S model that has four arms, each of which has a wider range of motion. The advantage of the fully robotic approach is that the robot is positioned over the patient’s left knee allowing access to the anorectal area should digital exam, vaginal retraction, or flexible sigmoidoscopy be required during the dissection.
Indications for robotic LAR include T1 through T3 tumors. Tumor location at any level of the rectum is possible, although strategy for intestinal reconstitution may differ. For tumors of the rectum 2 cm above the anorectal ring a double-stapled anastomosis is the preferred option. Tumors less than 2 cm from the anorectal ring that demonstrate no sphincter invasion are amenable to an intersphincteric resection. For this technique, the transanal intersphincteric dissection is performed first followed by the robotic transabdominal steps of the procedure. The specimen may be retrieved transanally or through a minilaparotomy incision followed by either a hand-sewn anastomosis or a double-stapled anastomosis. Rectal carcinomas invading the anal sphincter are surgically treated with abdominoperineal resection and permanent colostomy.
Contraindications to robotic LAR are the same as for laparoscopy. Body mass index (BMI) is not a restriction and those patients undergoing preoperative chemoradiation therapy are potentially appropriate candidates. A significant history of multiple previous operations may be a relative contraindication. If significant adhesions are encountered, lysis of adhesions may be laparoscopically completed prior to docking the robot. The robot is not as adept at moving to multiple quadrants of the abdomen because of vertical limitations on range of motion of the robotic arms.
Surgeon experience plays an important role in the success of robotic LAR and in ensuring both patient safety and good oncologic outcome. The surgeon should have experience with rectal cancer surgery and good knowledge of the open techniques. He/she should demonstrate advanced laparoscopic skills and be proficient in basic robotic skills that have a learning curve. These skills include the following:
Docking and undocking using the various arm and port clutches to move the arms
Maximizing space between the robotic arms and strategic planning to avoid arm collisions
Learning the console controls associated with the robotic system
Learning the various robotic instruments that differ from the laparoscopic instruments
Learning to use visual cues when manipulating the bowel and mesentery without haptic feedback and with forces that are motion scaled and electronically enhanced
Contraindications to robotic LAR procedures are the same as for the laparoscopic approach. Application of all minimally invasive procedures should be tailored to the level of the surgeon experience. Minimally invasive approaches to rectal cancers are technically challenging and require advanced skills for good technique and trouble-shooting. For robotic surgery, technical confidence can be overestimated during the training period since those surgeons without minimally invasive experience may find it easier to navigate the pelvis with the more stabilized system, accommodating instruments, smoother dissections, and magnified views that the robot offers.
Routine preoperative staging should be performed for all rectal cancers including colonoscopy, biopsy, CT scan, and either endorectal ultrasound or pelvic MRI. Oncology consultation may be appropriate for initiation of neoadjuvant chemoradiation therapy.
The robotic hybrid procedure involves three steps:
Laparoscopic mobilization of the left colon and splenic flexure, ligation of the mesenteric vessels (This mobilization can be performed medial to lateral or lateral to medial based on surgeon preference)
Robotic TME (The robot is positioned between the patient’s legs)
Specimen retrieval and anastomosis
The fully robotic procedure involves four steps. The robot is not moved during the procedure but the arms are undocked and redocked during the different phases.
Robotic vessel division and retroperitoneal dissection medial to lateral (The robot positioned over the patient’s left leg to reach from the pelvis to the left upper quadrant)
Mobilization of the splenic flexure
Robotic TME
Specimen retrieval and anastomosis
For both approaches, the robotic TME is followed by specimen retrieval, possible anastomosis, possible diverting loop ileostomy depending on the surgical plan.
Operating Room Set Up
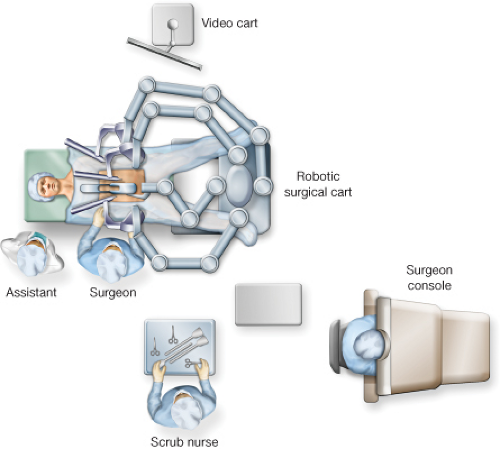
For the Hybrid Procedure (Fig. 17.1)
Assistant and scrub tech are positioned to the patient’s right.
The robot is positioned at the patient’s feet during the laparoscopic portion and then brought between the patient’s leg during the robotic TME.
The video cart and additional monitors are placed to the patient’s left.
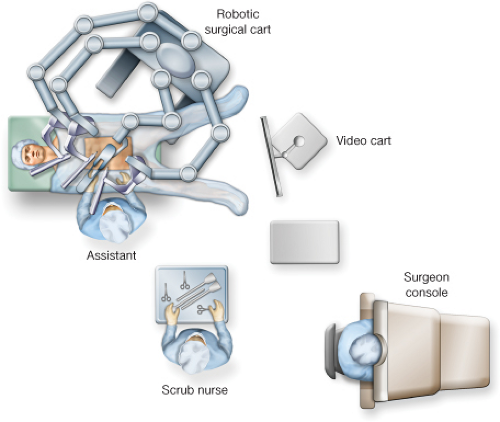
For the Fully Robotic Procedure (Fig. 17.2)
The assistant and scrub tech are to the patient’s right.
The video cart is at the foot of the bed.
The robot is positioned over the patient’s left leg.
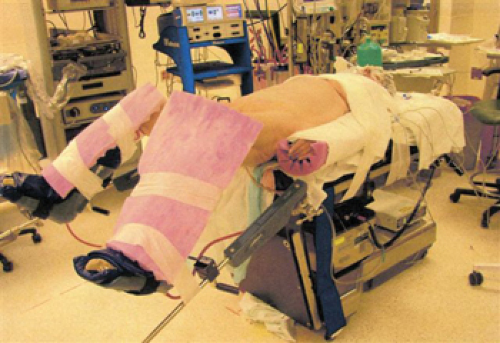
Patient Positioning (Fig. 17.3)
The patient is placed supine in a modified lithotomy position with the legs in padded adjustable stirrups. Both arms are tucked at the patient’s sides and the patient should be secured to the bed to avoid shifting in the Trendelenburg position. Towels are placed in an x-shaped fashion across the patient’s chest and tape is placed over the towels to secure the patient to the bed. For the hybrid procedure, both patient’s legs should be padded anteriorly to prevent injury from the robotic arms. For the fully robotic procedure, additional padding should be placed on the left leg. Placement of ureteric stents (optional) followed by foley catheter is performed prior to initiation of the robotic procedure. During the robotic portion of the procedure, the patient is placed in steep Trendelenburg with a 30-degree right lateral rotation to keep the small bowel out of the pelvis and in the right upper quadrant.
Port Placement and Docking
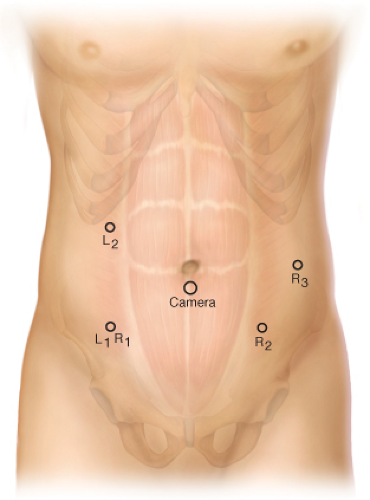
Hybrid Procedure Ports (Fig. 17.4)
The 12-mm camera port is placed 3 cm either above or below umbilicus midway between the xiphoid and the symphysis pubis.
The right lower quadrant port is both L1 (laparoscopic port 1) and R1 (robotic port 1). This should be a disposable 12-mm port (to accommodate a stapler) through which is telescoped an 8-mm nondisposable metal robotic port (for the robotic portion of the procedure).
The right upper quadrant port is L2. This port is a disposable 5-mm port.
The left lower quadrant port is R2, with additional port R3 placed left lower quadrant lateral to R2 if a Da Vinci S system (four arms) is used. Both of these ports are the robotic metal nondiposable ports.
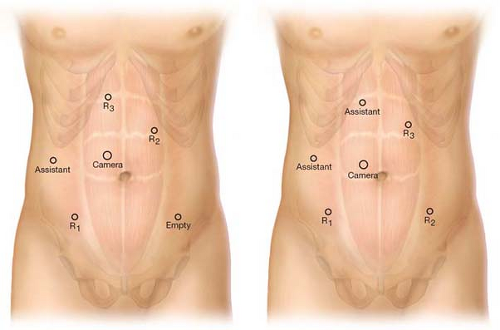
Fully Robotic Procedure Ports: Starting with Camera Port and then Clockwise (Fig. 17.5)
Camera port 12 mm placed 3 cm to the right and 3 cm above umbilicus.
R1: 12-mm port right lower quadrant (midclavicular line) through which is telescoped an 8-mm robotic port. The 8-mm port can be removed to place an endostapler.
Assistant port right lateral midabdomen: 5-mm port for retracting and suctioning by assistant.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree