Bismuth triple therapy was the first effective Helicobacter pylori eradication therapy. The addition of a proton pump inhibitor helped overcome metronidazole resistance. Its primary indication is penicillin allergy or when clarithromycin and metronidazole resistance are both common. Resistance to the primary first-line therapy have centered on complexity and difficulties with compliance. Understanding regional differences in effectiveness remains unexplained because of the lack of studies including susceptibility testing and adherence data. We discuss regimen variations including substitutions of doxycycline, amoxicillin, and twice a day therapy and provide suggestions regarding what is needed to rationally and effectively use bismuth quadruple therapy.
Key points
- •
Bismuth quadruple therapy, consisting of a proton pump inhibitor, bismuth, metronidazole, and tetracycline, is a good alternative first-line therapy and is especially useful when penicillin cannot be used or when clarithromycin and metronidazole resistance is common.
- •
The literature is confusing because bismuth quadruple therapy is used to denote regimens that differ greatly in terms of duration, doses, and administration in relation to meals.
- •
Proton pump inhibitors can help negate the deleterious effects of metronidazole resistance in bismuth quadruple therapy. The optimum dose of proton pump inhibitor is unclear. A double dose twice a day is recommended.
- •
In the presence of metronidazole resistance, the optimum duration is 14 days along with 1500 to 1600 mg of metronidazole in divided dosages. The optimum doses and dosing intervals for tetracycline and bismuth are as yet unclear.
- •
Poor patient adherence is a major issue with bismuth quadruple therapy. Patient education and counseling regarding the goals of therapy, the side effects, and the necessity to complete the full 14 days should be provided.
- •
As with all therapies, the decision to use bismuth quadruple therapy should be guided by the regional, local, and patient-specific antimicrobial resistance patterns and knowledge about effectiveness locally.
- •
Twice-a-day dosing may provide a high cure rate with fewer side effects, accomplishes a reduction in total antibiotic dose, and improves adherence. However, its effectiveness in relation to metronidazole resistance remains unclear.
Background
Eberle, in 1834, noted that bismuth, primarily as the white oxide, was introduced into medicine in 1697 by Jacobi and that its use was later popularized by Drs Odier of Geneva and De la Roche, of Paris. Throughout the nineteenth century, bismuth salts were widely and successfully used in gastroenterology. Bismuth continued to be used as a primary or adjuvant therapy for dyspepsia and peptic ulcer until being replaced successively by antacids, histamine-2 receptor antagonists, and proton pump inhibitors (PPIs). Bismuth also had a long history of use as an antimicrobial especially for the treatment of syphilis. In the United States, bismuth subsalicylate (eg, as Pepto-Bismol) was also used for dyspepsia and diarrhea and later to treat and prevent travelers’ diarrhea. In travelers’ diarrhea, bismuth was shown to function as a topical antimicrobial, thus linking its use as an anti-infective to its subsequent use for treatment of Helicobacter pylori -related peptic ulcer disease.
The most widely used forms of bismuth in use for gastroenterology at the time of the discovery of H pylori were bismuth subnitrate, subsalicylate, and subcitrate. In the 1970s, Gist-Brocades introduced a proprietary preparation of colloidal bismuth subcitrate (De-Nol) as an antiulcer therapy. The original De-Nol formulation was a colloidal suspension in ammonia water and had the very pungent odor of ammonia. The 1970s were also a time of great interest in ulcer pathogenesis and ulcer treatments. Many groups were also active in the study of ulcer in experimental animals. Colloidal bismuth subcitrate was shown to be able to coat and thus potentially protect the ulcer base, a property not seen with other bismuth preparations. Over time, the list of its properties potentially important in the treatment of peptic ulcer grew large ( Box 1 ).
- •
Bactericidal effect on H pylori
- •
Binding to the ulcer base
- •
Inactivation of pepsin
- •
Binding of bile acids
- •
Stimulation of prostaglandin biosynthesis
- •
Suppression of leukotriene biosynthesis
- •
Stimulation of complexation with mucus
- •
Inhibition of various enzymes
- •
Binding of epithelial growth factor
- •
Stimulation of lateral epithelial growth
Background
Eberle, in 1834, noted that bismuth, primarily as the white oxide, was introduced into medicine in 1697 by Jacobi and that its use was later popularized by Drs Odier of Geneva and De la Roche, of Paris. Throughout the nineteenth century, bismuth salts were widely and successfully used in gastroenterology. Bismuth continued to be used as a primary or adjuvant therapy for dyspepsia and peptic ulcer until being replaced successively by antacids, histamine-2 receptor antagonists, and proton pump inhibitors (PPIs). Bismuth also had a long history of use as an antimicrobial especially for the treatment of syphilis. In the United States, bismuth subsalicylate (eg, as Pepto-Bismol) was also used for dyspepsia and diarrhea and later to treat and prevent travelers’ diarrhea. In travelers’ diarrhea, bismuth was shown to function as a topical antimicrobial, thus linking its use as an anti-infective to its subsequent use for treatment of Helicobacter pylori -related peptic ulcer disease.
The most widely used forms of bismuth in use for gastroenterology at the time of the discovery of H pylori were bismuth subnitrate, subsalicylate, and subcitrate. In the 1970s, Gist-Brocades introduced a proprietary preparation of colloidal bismuth subcitrate (De-Nol) as an antiulcer therapy. The original De-Nol formulation was a colloidal suspension in ammonia water and had the very pungent odor of ammonia. The 1970s were also a time of great interest in ulcer pathogenesis and ulcer treatments. Many groups were also active in the study of ulcer in experimental animals. Colloidal bismuth subcitrate was shown to be able to coat and thus potentially protect the ulcer base, a property not seen with other bismuth preparations. Over time, the list of its properties potentially important in the treatment of peptic ulcer grew large ( Box 1 ).
- •
Bactericidal effect on H pylori
- •
Binding to the ulcer base
- •
Inactivation of pepsin
- •
Binding of bile acids
- •
Stimulation of prostaglandin biosynthesis
- •
Suppression of leukotriene biosynthesis
- •
Stimulation of complexation with mucus
- •
Inhibition of various enzymes
- •
Binding of epithelial growth factor
- •
Stimulation of lateral epithelial growth
Bismuth in the era of new concepts regarding pathogenesis and treatment of peptic ulcer
In the mid-twentieth century, peptic ulcer and its complications were a major medical problem in western countries. The importance of peptic ulcer was illustrated by the awarding of a Nobel prize to James Black in 1988 for the discovery of the histamine-2 receptor antagonists (in 1972) and for β-blockers (in 1964). The late 1970s and early 1980s saw the introduction of many new antiulcer agents (eg, sucralfate, histamine-2 receptor antagonists, synthetic prostaglandins, a tablet formulation of De-Nol, and finally, the PPIs). An epidemic of bismuth neurotoxicity occurred in France, which led to the removal of bismuth from many countries. However, in Europe, considerable interest in colloidal bismuth subcitrate continued based on studies showing that both endoscopically and histologically ulcer healing was more complete and recurrences less frequent following treatment with De-Nol compared with other agents. One particularly important comparison of liquid colloidal bismuth subcitrate and cimetidine randomized 46 patients with active duodenal ulcers. Forty patients (25 cimetidine, 15 bismuth) had symptomatic follow-up and 39 had endoscopic follow-up for up to 1 year. Ulcer relapse confirmed by endoscopy occurred in 79% of the cimetidine group compared with 27% of those receiving bismuth. Most importantly, H pylori was present at follow-up in 100% of those who received cimetidine, whereas 10 of the 15 receiving bismuth (43%) showed healing of gastritis, elimination of H pylori , and no ulcer relapse at the 1-year follow-up. At that time, peptic ulcer disease was thought to be incurable; “once an ulcer, always an ulcer” was the current dogma. Those results showed that the natural history of ulcer might be changed and provided a potential biologic basis for observation of a reduced rate of ulcer relapse following ulcer healing with bismuth subcitrate. It also was a harbinger of the results of subsequently published randomized studies proving that H pylori eradication prevented duodenal and gastric ulcer recurrences among those not also taking nonsteroidal anti-inflammatory drugs.
The initial trials of bismuth as an antimicrobial therapy for H pylori eradication reported H pylori eradication rates ranging from 10% to 30%. However, the duration of those early experiments were typically short and subsequent experience has suggested that these were possibly overestimated or changes in the formulation of bismuth subcitrate (eg, from liquid to tablet) may have reduced its effectiveness. Because the liquid formulation was not well accepted by patients, it was replaced by a swallow tablet produced by spray drying the liquid preparation. The fact that long-term follow-up of duodenal ulcer recurrence identified late recurrences and loss of statistical significance compared with H 2 -receptor antagonists is consistent with current notions that bismuth alone rarely cures H pylori infections and that all currently available preparations are similar in anti- H pylori effect. However, head-to-head comparisons of bismuth preparation alone or part of multidrug therapy are generally lacking. As noted above, following the epidemic of bismuth neurotoxicity in France, many countries removed all bismuth preparations from their pharmacopeias, and thus, bismuth compounds are not universally available for the treatment of H pylori infections. When used short term as for anti- H pylori therapy, bismuth therapy has proven to be both safe and effective.
Bismuth quadruple therapy for H pylori eradication
Starting in the mid to late 1980s, there were numerous clinical trials attempting to cure H pylori infections. When a single antibiotic proved ineffective, dual- and triple-drug therapies were tried, and eventually an effective regimen consisting of bismuth subcitrate, tetracycline, and metronidazole was identified by Tom Borody and colleagues in Australia. That original study used bismuth subcitrate 120 mg and tetracycline 500 mg, both 4 times a day, for 28 days and metronidazole 200 mg 4 times a day for 14 days. They reported a success rate of 94 of 100 subjects. Subsequently, metronidazole resistance was found to reduce the regimen’s effectiveness. However, the addition of a PPI proved to enhance its effectiveness irrespective of the presence or absence of metronidazole resistance. The regimen, often called bismuth quadruple therapy, consists of a PPI, a bismuth, metronidazole, and tetracycline. The dosages, duration of therapy, and administration in relation to meals differ as discussed later.
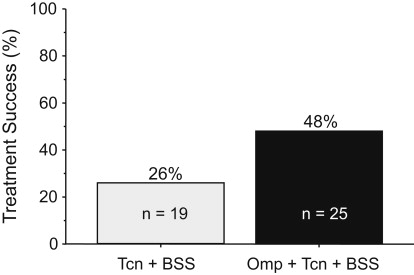
Antimicrobial resistance generally renders that particular antibiotic ineffective for resistant infections (eg, it functionally drops out of the regimen). The fact that the addition of a PPI appeared to negate the effect of metronidazole resistance suggested that metronidazole was possibly unnecessary and that the combination of a PPI, bismuth, and tetracycline components might suffice. That hypothesis was examined in 44 patients with peptic ulcer disease who received either tetracycline 500 mg and bismuth subsalicylate (Pepto-Bismol) 2 tablets both 4 times a day with or without omeprazole 40 mg in the am for 14 days. The overall cure rate was 48%, which was clinically unacceptably low. However, the addition of omeprazole was able to approximately double the efficacy of tetracycline and bismuth dual therapy ( Fig. 1 ).
Even today, it remains unclear how the PPI helps overcome metronidazole resistance. Metronidazole is a prodrug activated by enzymes within the bacterial cell. Resistance as assessed in vitro is associated with inactivation of one or more of those enzymes. Part of its effectiveness may be topical, and the concentration of metronidazole present in the stomach is very high, suggesting that as yet recognized enzyme pathways in the bacterial cell remain or become active at increased pH. The ability to partially overcome resistance is both metronidazole-dose and treatment-duration dependent. It is also unclear whether the ability to overcome the effect of metronidazole resistance is largely restricted to bismuth-containing regimens or even to bismuth–tetracycline-containing regimens. The HOMER study examined omeprazole, metronidazole, and amoxicillin (ie, no bismuth) and also showed an overall effect of dose and duration on effectiveness, but the benefit was not directly related to improved outcomes with resistant strains.
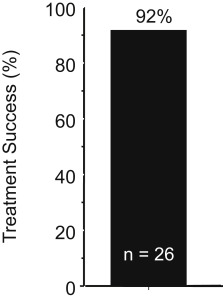
When H pylori -infected subjects with pretreatment-confirmed metronidazole-resistant infections received a 14-day bismuth quadruple regimen consisting of tetracycline 500 mg and bismuth subsalicylate (Pepto-Bismol) 2 tablets both 4 times a day with meals, plus metronidazole 500 mg 3 times a day and omeprazole 20 mg in the am , the cure rate was 92% ( Fig. 2 ), which was approximately what was expected in a metronidazole-susceptible population (there was no control group). This direct experiment confirms that the addition of a PPI could partially or possibly completely negate the deleterious effects of metronidazole resistance in bismuth quadruple therapy. Another experiment with 43 metronidazole-resistant strains (77% with minimal inhibitory concentration [MIC] ≥256 by Etest) reported a per-protocol cure rate of 92.1% (81.4% intertion to treat [ITT]). Two subjects dropped out after 7 days and both failed therapy. A large study from China found a large population of subjects with multidrug-resistant H pylori (susceptible to amoxicillin and tetracycline) and reported that among 101 patient with metronidazole-resistant strains 14-day bismuth quadruple therapy cured 93.1% per protocol, which was similar to the effectiveness of bismuth quadruple therapy, where amoxicillin was substituted for metronidazole (ie, 94.6% of 93 subjects per protocol).
The effect of metronidazole resistance as examined by meta-analysis
Several meta-analyses involving bismuth quadruple therapy have focused on understanding the results in terms of antimicrobial resistance (eg, ). The beneficial effect of adding a PPI in relation to metronidazole resistance was confirmed in an analysis of 93 studies (10,178 participants) that showed that metronidazole resistance reduced efficacy of bismuth, metronidazole, and tetracycline therapy (of different durations and dosing) on average by 26%. The reduction was only 14% following the addition of a gastric acid inhibitor, and it was concluded that even in areas with a high prevalence of metronidazole resistance, the quadruple regimen plus a PPI eradicated more than 85% of H pylori infections when given for 10 to 14 days.
Calculation of the effectiveness of bismuth quadruple therapy
In most western countries and in Korea and China, the an unsatisfactory outcome with bismuth quadruple therapy (eg, <90% or <85% treatment success per protocol) can be identified if one examines the duration of therapy, the doses used, patient adherence to the regimen, and whether metronidazole resistance was present. With clarithromycin-containing triple and quadruple therapies, the outcome can be reliably predicted provided one has data concerning the effectiveness of that therapy in relation to antimicrobial resistance (ie, with susceptible strains and with resistance with each individual antimicrobial and with combinations of antimicrobials). Similar calculations can theoretically be made for bismuth quadruple therapy based on the duration and the effectiveness of therapy in the presence of metronidazole resistance. Unfortunately, there is a paucity of data even from western countries and essentially none from areas where tetracycline resistance is likely to be an issue (eg, Iran or Turkey).
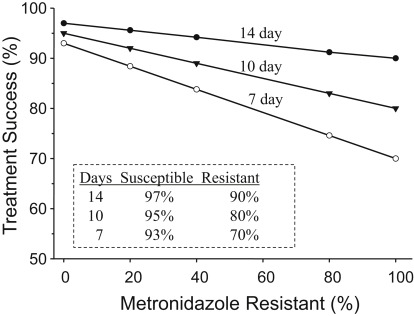
The data for Fig. 3 were derived empirically from the results of clinical trials but provides a reasonably accurate estimation in areas where bismuth quadruple therapy produces 90% or greater results with 14-day and full-dose therapy. The formula is ([success rate with susceptible strains times proportion with susceptible strains] + [success rate with resistant strains time proportion with resistant strains] = outcome per protocol). For example, for a 7-day regimen with 25% resistant strains (ie, 75% susceptible), the result would be (75 × 93%) + (25 × 70%) or ∼87% per protocol. It shows that the population result (per protocol) is expected to drop to less than 90% with 10-day therapy with 40% metronidazole resistance or less than 90% with 7-day therapy with 20% metronidazole resistance. Intention-to-treat results would be expected to be somewhat lower. The cure rate of those individuals with resistant strains receiving 7- and 10-day therapy would be approximately 70% and 80%, respectively, suggesting that if metronidazole resistance is possibly present and 14-day therapy would be the most prudent recommendation. Poor adherence (eg, the patient taking only 5 days of a 14-day prescription) would have a marked effect on outcome and, as side effects are common, poor adherence is a major issue with bismuth quadruple therapy. Another factor that would influence the outcome is the method used to assess metronidazole resistance. Assessment by Etest tends to overestimate the presence of metronidazole resistance. For example, in one study, 20 of the 37 subjects tested (54%) were judged to be metronidazole resistant by Etest and only 12 were confirmed by agar dilution. In a large study, the change in susceptibility result was approximately 17%. The authors recommend that for clinical trials, all resistance results with Etest be confirmed with agar dilution.
Adherence (compliance) with bismuth quadruple therapy
Adherence to the Protocol
Many anti- H pylori regimens are somewhat complicated and require taking drugs 2 to 4 times daily. In order to achieve the high degrees of success reported in clinical studies, it is important that both the clinician and the patient adhere to the details of successful protocols. Arbitrary changes in the protocol (eg, dose, duration, formulation) are similar to making arbitrary changes in a published recipe for French bread and then being surprised when the product is less than anticipated. Fig. 4 shows the outcome (ITT) of the bismuth quadruple therapy arms of recent comparative trials with legacy triple therapy. When one examines the details of the studies, one finds that what was called bismuth quadruple therapy often differed in terms of doses given. These studies used either 7 or 10 days to be the same as the triple therapy despite the evidence that all of these regimens do better with 14 days.
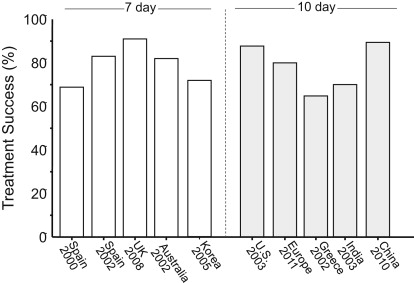
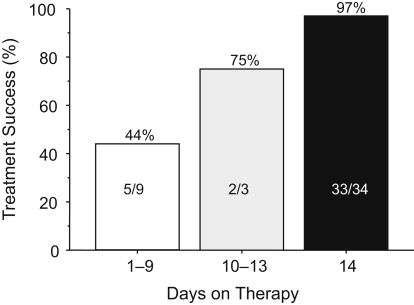
From the first part of this article, one would expect outstanding results from bismuth quadruple therapy, and here, neither 7- nor 10-day regimens provided acceptably high intention-to-treat success. An example is a 14-day treatment trial using a formulation in which the antibiotics and bismuth are contained within the same capsules (ie, Pylera) and omeprazole 20 mg 2 times a day. Forty-seven subjects were entered and 12 (25.5%) failed to take the planned 14 days of therapy. The most common reason for early stopping was the presence of an adverse event. As would be expected, treatment success increased as the duration of therapy increased ( Fig. 5 , Table 1 ). The cure rate ITT was 70% and per protocol (PP) was greater than 95%. All those with metronidazole-resistant strains who completed therapy were cured.
| Days Therapy Completed | Status | Total | Reason Stopped | |
|---|---|---|---|---|
| Infected | Eradicated | |||
| 2 | 2 a,b | 0 | 2 | Adverse events |
| 3 | 1 a | 0 | 1 | — |
| 6 | 0 | 2 | 2 | Adverse event |
| 7 | 1 b | 0 | 1 | Adverse event |
| 8 | 0 | 2 | 2 | — |
| 9 | 1 a | 0 | 1 | Adverse event |
| 10 | 0 | 0 | 0 | — |
| 11 | 1 b | 0 | 1 | Protocol violation |
| 12 | 0 | 1 | 1 | — |
| 13 | 0 | 2 | 2 | — |
| 14 | 1 a | 33 | 34 | Completed study |
| Total | 7 | 40 | 47 | — |
Soon after the introduction of bismuth, metronidazole, and tetracycline therapy, it became apparent that side effects were common; side effects tend to cause a reduced adherence to the regimen prescribed. With the exception of temporary discoloration of the tongue, bismuth is generally an innocuous medication when used for term use therapy. In contrast, tetracycline and metronidazole either separately or together frequently are associated with complaints. As part of the initial development of the regimen, Borody and colleagues examined whether increasing the frequency of administration without increasing the dosages would improve adherence. They compared two 14-day regimens of 4 versus 5 drug administrations per day (between 7 am and 11 pm ). The doses used were 108 mg of bismuth subcitrate in both arms, 500 mg of tetracycline 4 times a day versus 250 mg 5 times daily, and 250 mg of metronidazole 4 times a day versus 200 mg 5 times per day (ie, the total amount of tetracycline was reduced from 2 g to 1.25 g; the dose of metronidazole was 1 g but was less per dose. The antisecretory agent was ranitidine 300 mg at night; resistance was presumably rare). The per-protocol cure rate was slightly higher with the 5-day regimen (96% vs 92%, P = .07) ( Fig. 6 ). More importantly, side effects were significantly reduced ( P <.001), suggesting that it is possible to provide a more acceptable regimen. de Boer, who performed many of the initial Dutch trials examining dose, duration, and the use of PPIs, used a 7-point approach of patient education/motivation starting with taking time to talk to the patient and explaining the rationale. He then went on to explain the potential difficulties and side effects that might occur and provided details about how to take the medications. He and Borody had good but not perfect adherence. It was previously shown that structured counseling and follow-up resulted in improved outcomes with clarithromycin triple therapy. Their results are likely applicable to all H pylori therapies.
One approach to enhance compliance is to enhance convenience. Triple therapy has long been available in dose packs. The US commercial formulation of bismuth quadruple therapy was also formulated in a convenience pack (Helidac) and the newer formulation Pylera is prepackaged into capsules containing bismuth subcitrate potassium, metronidazole, and tetracycline. None of these packs have proven to improve adherence or result in a clear reduction in side effects. Studies of compliance have not shown that reduced adherence was related to the number of tablets per day required for H pylori quadruple therapy. The number of tablets or capsules per day can also be reduced in traditional therapy by using 500 mg per capsule of tetracycline and of metronidazole and bismuth 2 times a day (eg, ) to be equal to or less than that needed for current prepackaged quadruple therapy products.
All H pylori regimens are associated with side effects. A meta-analysis recently compared the side effects of bismuth quadruple therapy with other common H pylori regimens and reported that side effects were not greater with the exception of the cosmetic event, black stools. This meta-analysis confirmed the results of prior meta-analyses. Despite these encouraging words, those taking care of patients with H pylori recognized that all the regimens have a high incidence of side effects and that failure to motivate the patients regarding finishing the regimen often results in dropouts, and in clinical studies, loss to follow-up that can likely be reduced by patient education.
How to make bismuth quadruple therapy more acceptable
As noted previously, Borody and colleagues attempted to reduce the side effects by changing the frequency of dose administration and the doses with some success. The current standard regimen consists of a PPI 2 times a day, tetracycline 500 mg 4 times a day, at least 1500 of metronidazole, and bismuth 4 times a day. Shorter durations have been recommended, but this is generally a marketing ploy because bismuth quadruple therapy is generally reserved for patients in whom metronidazole resistance is likely, and duration and doses are important for excellent outcome. Although 14 days of full-dose therapy might be ideal, duration and dose of metronidazole have not been systematically examined in relation to metronidazole resistance. There are in fact some data suggesting that the doses considered ideal may be more than necessary. For example, there are several trials in which bismuth quadruple therapy was administered twice rather than 3 or 4 times a day ( Table 2 ). The authors show data from 11 arms and 9 studies (see Table 2 ). Cure rates for 10- to 14-day studies were more than 86% PP except in one instance, where a low-dose PPI was used. Seven days appeared to be possibly inferior to 10 or 14 days. Drugs included bismuth subcitrate and bismuth subsalicylate, 1 to 1.5 g of tetracycline, and 800 to 1000 mg of metronidazole along with full-dose PPI except in the one instance, where lansoprazole 15 mg 2 times a day was used. Studies were done in high (China, Turkey, and Italy [Sardinia]) and moderate metronidazole resistance areas (Unites States). Clearly, comparative studies are needed to assess efficacy as well as side effects in comparison to 3 and 4 times daily bismuth quadruple therapy and to address whether am and pm and noon and pm administrations are equivalent. In these studies, drugs were given with or after meals. The was also one study of 2 times a day omeprazole 20 mg, amoxicillin 1 g as a substitute for tetracycline, tinidazole 500 mg, and bismuth subcitrate 240 mg given every 12 hours for only 7 days that achieved a PP cure rate of 86% (84.1% ITT) that was not followed up with another study of longer duration.
| Year | Location | Bismuth a | Tetracyc | Metro | Meals | PPI ∗∗ | Days | No. | PP% | ITT% | Ref. |
|---|---|---|---|---|---|---|---|---|---|---|---|
| 1997 | USA | BSS 524 b.i.d. | 500 b.i.d. | 500 b.i.d. | am , pm | L 15 b.i.d. | 10 | 46 | 75 | 70 | |
| 2002 | Italy | BSC 240 b.i.d. | 500 b.i.d. | 500 b.i.d. | Noon, pm | P 20 b.i.d. | 14 | 118 | 98 | 95 | |
| 2003 | Italy | BSC 240 b.i.d. | 500 b.i.d. | 500 b.i.d. | Noon, pm | P 20 b.i.d. | 14 | 71 | 97 | 93 | |
| 2004 | USA | BSS 524 b.i.d. | 500 b.i.d. | 500 b.i.d. | am , pm | R 20 b.i.d. | 14 | 37 | 92.3 | 92.3 | |
| 2006 | Italy | BSC 240 b.i.d. | 500 b.i.d. | 500 b.i.d. | am , pm | E 20 b.i.d. | 10 | 95 | 95 | 91 | |
| 2009 | China | BSC 220 b.i.d. | 750 b.i.d. | 400 b.i.d. | am , pm | P 40 b.i.d. | 7 | 43 | 82.9 | 79.1 | |
| 2009 | China a | BSC 220 b.i.d. | 750 b.i.d. | 400 b.i.d. | am , pm | P 40 b.i.d. | 10 | 45 | 90.9 | 88.9 | |
| 2010 | China a | BSC 220 b.i.d. | 750 b.i.d. | 400 b.i.d. | am , pm | P 40 b.i.d. | 10 | 85 | 91.6. | 89.9 | |
| 2011 | Italy | BSC 240 b.i.d. | 500 b.i.d. | 500 b.i.d. | Noon, pm | P 20 b.i.d. | 14 | 202 | 98 | 92 | |
| 2011 | Italy | BSC 240 b.i.d. | 500 b.i.d. | 500 b.i.d. | Noon, pm | P 20 b.i.d. | 10 | 215 | 95 | 92 | |
| 2013 | Turkey | BSC 600 b.i.d. | 500 b.i.d. | 500 b.i.d. | am , pm | O 20 b.i.d. | 14 | 38 | 86.8 | 73.3 | |
| 2005 | Iran | BSC 240 b.i.d. | 500 b.i.d. | 500 b.i.d | am , pm | O 20 b.i.d. | 14 | 76 | – | 76.3 | |
| 2006 | Iran a | BSC 240 b.i.d. | 750 b.i.d. | 500 b.i.d. | am , pm | O 20 b.i.d. | 3 | 40 | 54 | 50 | |
| 2006 | Iran a | BSC 240 b.i.d. | 750 b.i.d. | 500 b.i.d. | am , pm | O 20 b.i.d. | 7 | 41 | 45.9 | 41.4 | |
| 2006 | Iran a | BSC 240 b.i.d. | 750 b.i.d. | 500 b.i.d. | am , pm | O 20 b.i.d. | 14 | 40 | 40 | 35 |
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree







