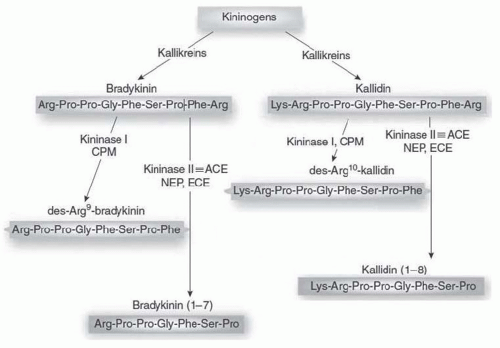
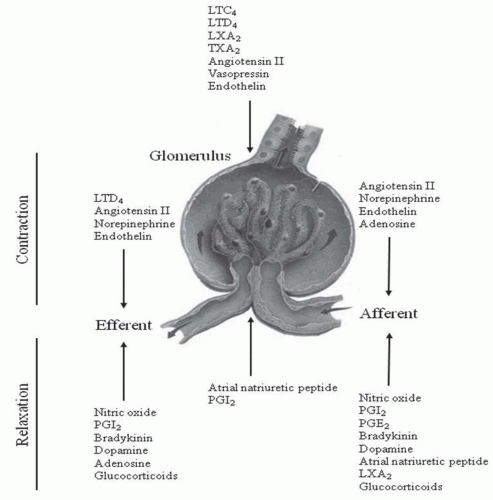
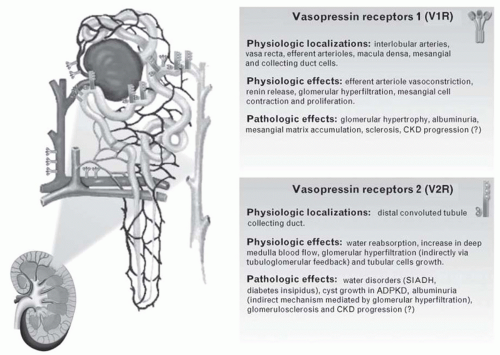
Physiology of Arginine Vasopressin in the Kidneys
The most sensitive stimulus for AVP secretion into the bloodstream is increased plasma osmolality. Osmosensitive neurons that respond to changes in plasma osmotic pressure by varying their intracellular water content have been identified in the anterior hypothalamus, specifically in the organum vasculosum of the lamina terminalis (OVLT) and the subfornical organ (SFO).
15 When stimulated by osmosensitive neurons, the magnocellular neurons release the stored AVP into the posterior pituitary (an area that lacks a blood-brain barrier) and AVP enters the general circulation. As little as 1% change in plasma osmolality leads to a change in AVP concentration that is sufficient to modify renal water excretion.
16 AVP secretion is almost completely suppressed when plasma osmolality decreases below an average of 280 mOsm per kg of water in humans.
17Secretion of AVP is also influenced by alterations in intravascular volume and blood pressure, sensed by baroreceptors located in the heart, aortic arch, and carotid sinus.
18 These signals are transferred through the vagal nerves to the
nucleus solitarius in the brainstem, from which postsynaptic pathways project to the magnocellular neurons. Whereas a 5% to 8% decrease in blood volume or systemic arterial pressure has little effect, further hemodynamic compromise leads to a steep increase in circulating AVP levels. Significant reductions (10%-30%) in circulatory arterial volume or blood pressure can override osmoregulation and result in markedly increased AVP levels in the face of decreased plasma osmolality.
19 Other less potent stimuli for AVP secretion include fever, emesis,
20 and oropharyngeal osmoreceptors.
21AVP circulates in the plasma nearly in an unbound form. Levels of circulating AVP depend on both the rate of AVP release from the posterior pituitary and the rate of AVP degradation. As discussed, the major factor controlling AVP release is plasma osmolality. The liver and the kidney both contribute to the breakdown of AVP and the decline in AVP levels when secretion ceases. In fact, the half-life of AVP in the circulation is 18 minutes due to rapid clearance by hepatic and renal vasopressinases.
22 Under physiologic conditions, plasma vasopressin concentrations vary with serum osmolarity between 0 to 5 pg per mL.
22It is noteworthy that AVP levels are difficult to measure in plasma because of the instability of this peptide and the low sensitivity of available AVP antibodies. Copeptin (or C-terminal proarginine vasopressin, CT-proAVP) is the C-terminal part of AVP, which is secreted stoichiometrically with AVP in a manner similar to C-peptide and endogenous insulin.
23 CT-proAVP provides a reliable means for estimating prevailing AVP levels in the circulation, thus facilitating the study of AVP in human diseases. A recent cohort study in renal transplant patients suggests that high CT-proAVP strongly correlates with a negative renal prognosis. In fact, in this study, the plasma concentrations of CT-proAVP predicted renal function loss over a 3.2-year follow-up.
24AVP exerts its biologic actions through three specific cell-surface AVP receptors identified as V
1R (also called V
1aR), V
2R, and V
3R (also V
1bR).
25 These receptors belong to the G protein coupled receptor superfamily (
Table 8.2). In the human kidney, mRNA for V
1Rs predominates in cortical collecting ducts (CCD), gradually decreasing as the collecting duct enters the medulla.
26 In addition, whereas V
1R mRNA is diffusely expressed in the CCD, it is restricted to the intercalated cells in OMCD. V
1Rs are responsible for mediating vascular smooth muscle cell vasoconstriction by activating G protein-dependent phospholipase C (PLC) and the downstream effectors, diacylglycerol (DAG), and inositol 1,4,5-triphosphate (IP
3). In turn, DAG stimulates protein kinase C (PKC), whereas IP
3 increases cytosolic Ca
2+, thus
initiating the second-messenger cascade responsible for the cellular actions of AVP.
25 Stimulation of V
1R, although not directly involved in control of tubular water and electrolyte transport, increases sodium excretion because of the influences on blood pressure, effective arterial circulating volume, glomerular filtration rate, and circulation in the vasa recta system.
27,
28 Additional biologic effects of AVP mediated through V1Rs include platelet aggregation
29 and increased glycogenolysis and gluconeogenesis in the liver.
30The V
3R (V
1bR), also coupled to PLC signaling, is present on neurons in the anterior pituitary (adenohypophysis) and is thought to mediate AVP-induced corticotropin secretion.
25,
31,
32 They are also found elsewhere in the brain, especially in the pyramidal neurons of the hippocampal CA2 field, in which they mediate fundamental physiologic actions such as memory and body temperature control as well as social behavior.
33 In addition, this receptor has been localized to pancreatic islet cells, modulating insulin secretion.
34 However, its presence and role at the level of the medulla in rat kidney remain unclear.
V
2Rs, on the other hand, are heavily expressed in the medullary TAL, macula densa (MD), connecting tubule, and cortical and medullary collecting duct, as well as weakly expressed in cortical thick ascending limb (TAL) and distal convoluted tubule.
35 These are the best characterized and studied vasopressin receptors. By binding to V
2R, AVP increases water reabsorption through multiple mechanisms.
31 Activation of V
2R results in increased cyclic adenosine monophosphate (cAMP) levels and activation of PKA, which promotes insertion of water channels into the luminal suface of the epithelial tubular cells.
36 This ultimately mediates the antidiuretic effect of AVP by allowing back diffusion of water down its concentration gradient.
37 In addition, V
2Rs modulate sodium reabsorption through the epithelial Na
+ channel (ENaC) across principal cells.
38,
39,
40,
41 This facilitates free water reabsorption by supporting the axial corticomedullary hyperosmotic gradient. It was also recently shown in rats that AVP modulates sodium reabsorption even in the distal convoluted tubule by acting on the thiazide-sensitive Na
+-Cl
– cotransporter (NCC).
42 NCC is important in defining sodium delivery to the collecting duct,
which is necessary for ENaC activity. Finally, V
2Rs activate urea transporters, such as UTA1, in the distal nephron.
43,
44,
45 This increase in urea reabsorption and recycling maximizes sodium reabsorption in the TAL by supporting the axial hyperosmotic gradient drawing water from the distal nephron.
46 The clinical importance of the V
2R in water balance disorders is underlined by the current use of V
2R antagonists in a clinical setting (see later).
It is noteworthy that AVP also binds to two nonspecific receptors: oxytocin receptors and P2 purinoreceptors. Oxytocin receptors are found in the breast, ovary, uterus, and hypothalamus. Since the affinity of this receptor for vasopressin is relatively low, the clinical effects of this hormone are limited under physiologic conditions.
47 AVP may also act on P2 purinoreceptors in the heart, causing coronary vasoconstriction and contributing to the reduction in cardiac output.
48
Water Channels
The discovery of the family of aquaporin water channels was crucial to the understanding of the mechanism by which AVP can increase water permeability in the kidney. The group of Peter Agre discovered the first aquaporin in human erythrocytes.
49 To date, seven different aquaporin (AQP) have been shown to be expressed in the human kidney and to be involved in renal water reabsorption.
50AQP2 is the vasopressin-sensitive water channel expressed in the principal cells of the collecting duct, where it shuttles between intracellular storage vesicles and the apical membrane.
51 Knocking out the AQP2 gene produces a severe concentration defect in these mice, resulting in postnatal death.
52 It has now been shown to be involved in many clinical disorders (see later). AQP1 is constitutively expressed on the basolateral and apical membrane of epithelial cells lining the proximal tubule and thin descending limb, as well as endothelial cells of the descending vasa recta. It not only plays a role in water reabsorption from urine in these segments but is also critical for a functional countercurrent multiplication system.
53 AQP3 and AQP4 are expressed on the basolateral membrane of the principal cells of the collecting duct, and they represent an exit pathway from these cells for water entering through AQP2.
54,
55 Similar to AQP1, AQP7 is expressed at the apical membrane of proximal tubules (S3 segment) and has been shown to mediate glycerol reabsorption in addition to water.
56 AQP6 is found in intracellular vesicles of acid-secreting α-intercalated cells in the collecting duct.
57 It is thought to be involved in urinary acid secretion. AQP11 is localized to the endoplasmic reticulum (ER) in the proximal tubule. Interestingly, knocking out the AQP11 gene in mice is fatal because of the onset of polycystic kidney disease.
58
Clinical Pathophysiologic Role of Arginine Vasopressin in the Kidneys
AVP is implicated in major clinical syndromes of alterations in water metabolism, namely nephrogenic diabetes insipidus (NDI) and the syndrome of inappropriate secretion of antidiuretic hormone (SIADH). In addition to water metabolism, it has been recently suggested that AVP may play a role in the initiation and the progression of chronic kidney disease (CKD) and in the most prevalent form of hereditary renal disease, namely the adult polycystic kidney disease (
Fig. 8.2).
59NDI is characterized by impaired AVP-induced water reabsorption, resulting in polyuria and polydipsia.
60 If water intake is inappropriate, patients with NDI may fail to thrive, suffer from mental retardation, and die early. NDI can be acquired such as following lithium treatment, hypokalemia, hypercalcemia, or ureteral obstruction, all of which lead to downregulation of AQP2.
61,
62,
63,
64 NDI can also be inherited (congenital).
65 In fact, two gene mutations have been linked to congenital NDI: one in the
AVPR2 gene encoding V
2R (X-linked NDI) or mutations in the
AQP2 gene (autosomal recessive or autosomal dominant NDI). More than 90% of patients with congenital NDI suffer from X-linked NDI. In both cases, patients cannot concentrate their urine despite normal or elevated plasma concentrations of vasopressin, leading to a massive loss of water through the kidney. So far, NDI is managed by salt restriction combined with hydrochlorothiazide diuretics to reduce urine output.
66 However, no cure is available so far.
Abnormal water handling of central origin includes SIADH in which AVP levels are abnormally elevated and not suppressed when plasma osmolality concentration falls below the osmotic threshold for physiologic AVP secretion.
67 SIADH is characterized by impaired water excretion in the absence of renal insufficiency, adrenal insufficiency, or any recognized stimulus for AVP secretion. Renal water retention and extracellular fluid expansion are compensated for by increased urinary Na
+ excretion leading to life-threatening hyponatremia. The most common causes of the syndrome of inappropriate AVP secretion are neoplasia, neurologic disorders, congestive heart failure, liver cirrhosis, preeclampsia, and drugs such as thiazide diuretics or selective serotonin reuptake inhibitor antidepressants.
67 Four patterns of AVP dysregulation in patients with SIADH have been observed.
68 The most common pattern (in ˜40% of patients with SIADH) is the excessive and unregulated release of AVP, which is unrelated to plasma osmolality. In the second most common pattern (˜30% of patients), referred to as “reset osmostat,” AVP release continues to regulate water excretion at a lower plasma osmolality set-point. Although most tumors (e.g., lung carcinoma) manifest the first type of SIADH, some also present with the second type, thus the pattern of abnormal AVP secretion cannot be utilized to predict the cause of SIADH. A third rare pattern is characterized by an inability to stop AVP secretion at low plasma osmolalities, but the osmoregulation of AVP is otherwise normal. This pattern may be due to dysfunction of inhibitory hypothalamic neurons, leading to persistent low-grade basal AVP secretion. In the fourth pattern, a rare clinical picture of SIADH, the normal osmoregulation of AVP secretion is not altered (˜10% of patients) but AVP levels are low or undetectable. It is thought that a nephrogenic SIADH
(NSIADH) may be responsible for this picture. In fact, in some children, it appears to be due to an activating mutation of V
2R. In other patients, it may be due to abnormal control of aquaporin-2 water channels in renal collecting tubules or production of an antidiuretic principle other than AVP.
69 In a study with SIADH patients, treatment with V
2R antagonists, commonly referred to as vaptans, increased serum Na
+ concentration and decreased its excretion. Tolvaptan has been recently approved in the United States and Europe for the treatment of hyponatremia associated with SIADH, as well as cirrhosis and congestive heart failure. Recently, a dual vasopressin V
1R and V
2R antagonist, conivaptan, improves hyponatremia in rats with SIADH, suggesting a therapeutic potential for conivaptan in the treatment of SIADH.
70AVP has also been shown to modify vascular tone in renal microvessels. Short-term infusion of AVP does not alter either renal blood flow or the GFR.
71 Alternatively, chronic AVP administration increases the intraglomerular capillary pressure and GFR through tubuloglomerular feedback.
72 In addition, a sustained increase in water intake and the consequent AVP suppression reduce proteinuria and the severity of glomerular and tubular damage in 5/6 nephrectomized rats.
73,
74 Thus, it seems likely that a chronic AVP-induced hyperfiltration may alter the glomerular barrier and start a series of events leading to enhanced protein loss and glomerulosclerosis. An increase in urinary albumin excretion represents an early predictor of glomerular damage in diabetes mellitus and a risk factor for cardiovascular complications in hypertension. Studies show that the Brattleboro rat, a model of central diabetes insipidus with complete lack of AVP, is protected from hyperfiltration, albuminuria, and renal hypertrophy after streptozotocin-induced diabetes mellitus.
75 This suggests that AVP plays a role in hyperfiltration and glomerular damage induced by diabetes. These observations are also of relevance to humans. A marked increase in AVP plasma levels is well documented in diabetes mellitus.
76 AVP through V
1R induces contraction of cortical efferent, but not afferent, arterioles. Administration of a V
1R selective antagonist to noninsulin-dependent diabetic patients modestly reduces albuminuria partly by decreasing intraglomerular capillary pressure.
77 Thus, although V
1Rs (but not V
2Rs) are downregulated in diabetes mellitus, they could mediate part of the increase in albumin excretion. Interestingly, administering desmopressin, a selective V
2R agonist, to healthy humans and patients with central diabetes insipidus significantly increases urinary albumin excretion, but this effect is absent in those with hereditary nephrogenic diabetes insipidus secondary to V
2R mutations.
78 These findings suggest that the AVP-induced rise in albuminuria depends on V
2Rs. This is further confirmed by the observation that plasma copeptin levels correlate with microalbuminuria
in the PREVEND study.
79 However, V
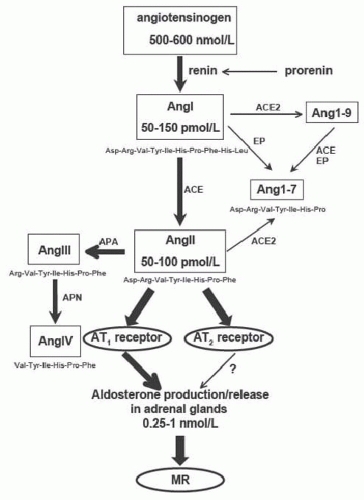
2 receptors have not been found in glomeruli or proximal tubules, suggesting indirect effects. Recent evidence suggests a strong interaction between AVP and the renin-angiotensin system (RAS).
80,
81 In fact, chronic RAS blockade by angiotensin-converting enzyme (ACE) inhibitors or angiotensin receptor blockers (ARBs) prevents the desmopressin-induced albuminuria, indicating that RAS mediates the effects of AVP on glomerular hemodynamics.
82 Recent studies using V
1R knockout mice show that AVP regulates body fluid homeostasis and the GFR by activating RAS through V
1Rs in MD cells, and subsequently the V
2R-aquaporin 2 system.
83 Simultaneous AVP and RAS blockade may represent a good therapeutic approach for delaying renal disease progression.
Another interesting observation is that mesangial cells express V
1Rs, which mediate AVP-induced cell contraction.
84 In addition, prolonged exposure to AVP promotes mitogenesis and proliferation of these cells, which ultimately leads to an increased accumulation of extracellular matrix, a pathologic feature found in various glomerular diseases.
85,
86 In fact, addition of AVP to cultured mesangial cells increases in a dose-dependent manner the synthesis and release of matrix proteins, such as type I and IV collagen, fibronectin, and transforming growth factor β.
87 In addition, AVP inhibited the synthesis of matrix metalloproteinase (MMP)-2, which degrades matrix proteins including type IV collagen, and stimulated endothelin (ET)-1 secretion from mesangial cells, another mitogenic factor.
88In the remnant kidney model in rat, a model of progressive CKD in humans, V
1R antagonists (but not V
2R) prevent proteinuria and glomerulosclerosis in the initial phases of disease, but have limited effectiveness in established renal damage.
89,
90 Similar observations were reported in 5/6 nephrectomized, salt-loaded spontaneously hypertensive rats (SHRs), in which the increase in urinary protein excretion and the progression of nephrosclerosis were attenuated with a V
1R antagonist but not with a V
2R antagonist.
91AVP has also been linked to autosomal dominant polycystic disease (ADPKD), an inherited disorder characterized by the development within renal tubules of innumerable cysts that progressively expand to cause renal insufficiency.
92 It has been shown that 3′-5′-cyclic adenosine monophosphate (cAMP) stimulates tubule cell proliferation and transepithelial fluid secretion, both of which contribute to enlarge renal cysts.
93 AVP operates continuously in ADPKD patients to promote cAMP production in the distal nephron and collecting ducts via V
2Rs, thereby contributing to cyst enlargement and renal dysfunction.
94 Studies in animal models of ADPKD provide compelling evidence that blocking AVP’s actions dramatically improves disease progression.
95,
96 This prompted the initiation of an international clinical trial
97 testing the efficacy of tolvaptan, a V
2R inhibitor, in the treatment of ADPKD.
98 Alternatively, a recent review discusses that the impact of simply increasing the amount of solute-free water drunk evenly throughout the day by patients with ADPKD on decreasing plasma AVP concentrations and mitigating the actions of cAMP on the renal cysts.
99In conclusion, in recent years, AVP has been implicated in the initiation and progression of many kidney diseases, playing a role beyond water metabolism. Further studies are required to determine whether AVP antagonists and/or AVP suppression by a high water intake can be useful for the treatment of nephropathies, from ADPKD to diabetic and nondiabetic CKD.