Vasectomy reversal has come a long way since the first anastomosis of the vas deferens and epididymis. Although its history is not as politically charged as that of vasectomy, the progress of reversal surgery has had its share of brilliant discoveries and missteps. In the early part of the twentieth century, vasovasostomy and vasoepididymostomy were esoteric procedures, but by the 1970s, a majority of urologists had some experience with reversal surgery. With the advent of microsurgical technique, reversal surgery has become once more a specialist’s undertaking. The history of vasectomy reversal is an excellent case study in the evolution of surgery.
With the application of vasectomy for eugenic, punitive, and therapeutic purposes gaining momentum in the early part of the twentieth century, a new surgical procedure, the vasectomy reversal, was born. The dawn of the vasectomy procedure was marred by dubious scientific and at times overtly sinister elements. Although the history of vasectomy is discussed in another article in this issue, a brief discussion of the development of the vasectomy procedure is pertinent to this discussion of vasectomy reversal. Vasectomies in the early 1900s were not as they are today, a safe and effective method of voluntary contraception; they often were performed for medically questionable reasons, such as severe prostatic hypertrophy and physical and mental revitalization through the Steinach rejuvenation procedure, and for political motives, such as eugenics and punitive sterilization of criminals. The medical principle of primum non nocere was not always followed in the early years of this procedure. The Nazi mass sterilization effort during World War II was merely the culmination of decades of medical demagoguery and misapplication of the vasectomy procedure.
Vincent J. O’Conor, Chicago urologist and former chairman of the Department of Urology at Northwestern University Medical School, posited that these political underpinnings were the spurs that promoted considerable interest in the vasectomy reversal procedure by the medical and nonmedical communities, including members of religious groups. As of 1948, however, O’Conor observed that most people assumed that reversal surgery was hardly worth considering, given the technical challenges and low success rates. Because urologic surgeons still approached this relatively untested reversal procedure with trepidation and because of the political, religious, and personal implications of vasectomy surgery, men undergoing vasectomy and its reversal often did so under a cloak of secrecy. Furthermore, the legality of this procedure was in question in most states, and urologists performing the surgery were at risk for legal challenges and malpractice suits. In his brief article, O’Conor provides a fascinating glimpse into the practice of male reproductive medicine in post–World War II society.
“Founding father of modern clinical andrology”
The birth of the reversal procedure goes back even further than O’Conor’s era. A history of surgical reversal rightfully begins with the work of Edward Martin, Chief Surgeon at the University of Pennsylvania during the early years of the past century, although technically he performed vasoepididymostomies in men who had obstruction secondary to epididymitis, not vasectomy ( Fig. 1 ). In 1902, Martin reported the first documented vasoepididymostomy in his study of 192 sterile couples and examination of sperm morphology. He initially performed the procedure on three dogs before operating on a man who had obstructive azoospermia. The following year, Martin reported his case of unilateral side-to-side vasoepididymostomy in a man who had a history of epididymitis and gonococcal urethritis, which resulted in sperm in the ejaculate and the birth of a full-term infant. After incising the epididymis at a location yielding exudation of milky fluid, Martin constructed the vasal-epididymal fistula using four fine silver wire sutures brought from the outer surface of the vas deferens into the lumen and then through the cut surface of the epididymis and its tunic ( Fig. 2 ). In 1909, Martin published his series of 15 azoospermic men who had obstructive lesions, 11 who had epididymal and 4 who had vasal obstruction. Possible origins of azoospermia in these men included orchitis (nonobstructive azoospermia), congenital absence of the vasa deferentia, secondary vasal atrophy, long vasal stricture, and distal vasa or ejaculatory duct obstruction. Martin performed vasoepididymostomies in the 11 men who had epididymal obstruction using the same technique described in his previous publication, with patency and pregnancy rates of 64% and 27%, respectively. Not only did Martin demonstrate the effectiveness of the reconstructive procedure for obstructive azoospermia but also he observed that azoospermia can be obstructive and nonobstructive. Martin’s clinical acumen was remarkable, and his observations seem to fit current standards of reproductive medicine rather than those of 1909. He even checked vasal patency during the vasoepididymostomy procedure, foretelling today’s use of vasography. The significance of his contributions to the field of male infertility inspired Jequier to entitle Martin the “founding father of modern clinical andrology” in her profile piece.

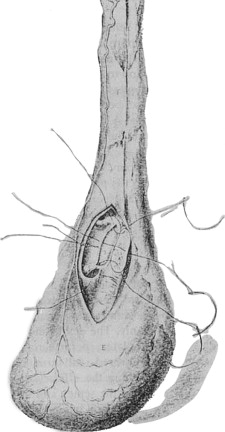
Francis Hagner of Washington, DC, was an early proponent of Martin’s surgery. In 1907, Hagner reported at the twenty-first annual meeting of the American Association of Genito-Urinary Surgeons two cases of successful anastomosis between the vas deferens and the globus major; one surgery had been performed by Martin and the other by Hagner. The surgery was performed with fine silver wire and curved intestinal needles, and the vas deferens was anastomosed to the epididymis after observation of white fluid exudation from a small wedge-shaped incision in the globus major. Sperm was detected in the ejaculate at 1 month. Hagner’s continued success with the vasoepididymal anastomosis helped popularize the procedure. In 1936 he reported patency and pregnancy rates of 64% and 48%, respectively, in 33 patients undergoing vasoepididymostomy. Hagner’s technique was modified and used by Hanley in Great Britain and Bayle in France.
Capacity for regeneration
H. C. Rolnick was another Chicago urologist at Northwestern University Medical School who contributed to the development of vasectomy and reconstructive surgery. In 1924, he published his series of 48 vasal surgeries in 25 dogs, in which he ligated, incised, or resected the vasa to determine their regenerative capacity. In the five dogs in which both vasa were ligated with catgut suture, all vasa were patent when checked after 21 to 38 days. In one of the dogs, the left vas deferens was sutured to the skin and a horsehair was left in the lumen, but this did not prevent patency. When Rolnick made multiple longitudinal and oblique incisions of the vasa, three of five were patent. Multiple transverse incisions of the vas always resulted in occlusion of the lumen. When six vasa were divided and separated from the sheath, however, no patency was observed. In contrast, six of seven vasa achieved patency when divided without disturbing the sheath or deferential vessels. Foreign body or suture in the lumen did not cause occlusion. In several dogs, Rolnick performed reversal surgery of the divided vasa and achieved patency in five of 13 anastomoses. From these results, he concluded that the vas deferens had the ability to resist trauma and restore its luminal patency and the intact vasal sheath and deferential vessels play an important role in the restoration of vasal integrity after injury.
Capacity for regeneration
H. C. Rolnick was another Chicago urologist at Northwestern University Medical School who contributed to the development of vasectomy and reconstructive surgery. In 1924, he published his series of 48 vasal surgeries in 25 dogs, in which he ligated, incised, or resected the vasa to determine their regenerative capacity. In the five dogs in which both vasa were ligated with catgut suture, all vasa were patent when checked after 21 to 38 days. In one of the dogs, the left vas deferens was sutured to the skin and a horsehair was left in the lumen, but this did not prevent patency. When Rolnick made multiple longitudinal and oblique incisions of the vasa, three of five were patent. Multiple transverse incisions of the vas always resulted in occlusion of the lumen. When six vasa were divided and separated from the sheath, however, no patency was observed. In contrast, six of seven vasa achieved patency when divided without disturbing the sheath or deferential vessels. Foreign body or suture in the lumen did not cause occlusion. In several dogs, Rolnick performed reversal surgery of the divided vasa and achieved patency in five of 13 anastomoses. From these results, he concluded that the vas deferens had the ability to resist trauma and restore its luminal patency and the intact vasal sheath and deferential vessels play an important role in the restoration of vasal integrity after injury.
Vasovasostomy
In 1919, Quinby reported the first successful vasovasostomy in a man who had undergone bilateral vas resection in 1911. He created the anastomosis over a strand of silkworm gut, which was removed after 10 days. Quinby’s assistant for this historic procedure was none other than O’Conor. O’Conor subsequently used Quinby’s technique in 14 vasectomized patients, resulting in a patency rate of 64%. In the same article published in 1948, O’Conor reported the results of his survey of 1240 urologists on the topic of vasectomy reversals. Seven hundred fifty urologists completed the questionnaire, and only 135 had any experience with the procedure. Of the 420 reported operations, patency rate was 38%, although the rate of spontaneous recanalization of the vasa was not determined. Several such surveys have been conducted in the ensuing decades, and this report provides the first snapshot of clinical practice patterns for vasectomy reversal surgery.
By the 1970s, many reports on macrosurgical techniques for vasovasostomy began appearing in the literature. Hulka and Davis reviewed vasovasostomy series from the United States, India, and Denmark and compiled 705 cases. They found a patency rate of 60% and a pregnancy rate of 44% in series reporting pregnancies. The investigators discussed pertinent anatomic considerations, such as the average luminal diameter of the vas deferens (0.55 mm) having significant variation and pondered whether or not ligation of the sympathetic nerve fibers of the inferior spermatic nerve during vasectomy resulted in permanent impairment of sperm transport through the vas deferens. They also reviewed methods for reversible vasocclusion, including use of prosthetic plugs with injections of Silastic and other nonreactive synthetic materials, an intra vas device similar in theory to the intrauterine device, and the vas clip and vas valve. Even within the confines of this scientific review article, hints of the broader political and social context of vasectomy and vasectomy reversal during the 1970s surfaced, with references to the zero population growth movement and allusions to the second wave of the feminist movement. Interest in vasectomy flourished during this time because of increased interest in family planning by men and the emancipation of women. With the increasing popularity of vasectomy, the relevance of vasectomy reversals inevitably followed.
In 1973, Getzoff published another questionnaire study of 150 urologists, examining their views and management of reversal surgery. On the topic of vasoepididymostomy, 13.3% had never performed this procedure, 28% had had no success, 20.7% rare (1%) success, 21.3% occasional (5%) success, 14% moderate (20%) success, and 2.7% moderately encouraging success (50% to 70% reported in the literature). When indications for a vasoepididymostomy were present, 9.3% urologists encouraged the operation, 27.3% discouraged it, and 63.3% had discussions with the patients about the procedure and prognosis. The same questions were asked regarding vasovasostomy, and 6% had never performed this procedure, 8% had had no success, 11.3% rare (1%) success, 20% occasional (5%) success, 38.7% moderate (20%) success, and 16% encouraging success (50% to 70% reported in the literature). When a patient presented for a potential vasovasostomy, 8.7% urologists encouraged the operation, 5.3% discouraged it, and 86% had discussions about the procedure and prognosis. Sixty-two percent believed a second procedure should not be attempted if the initial reversal surgery failed. Regarding surgical technique, no splint was used in 9.9% of cases, silver wire in 51.1%, nylon suture in 33.8%, Silastic tubing in 9.5%, steel wire in 3.2%, silk suture in 0.8%, and other splints in 1.6%. Compared with the results from O’Conor’s survey in 1948, there was a dramatic increase in the proportion of urologists who had at least some experience with reversal surgery. Perhaps more telling is the stark contrast between the published success rates in the literature (50% to 70%) and the low success rates in actual clinical practice.
Etiologies of vasectomy reversal failure
Since the early studies of Rolnick, several groups have studied the causes of reversal surgery failures. Failures can be functional or anatomic.
Functional Failure: Agglutinating Antibodies
Sullivan and Howe tested the serum of 45 men who had sperm in their ejaculate after reversal surgery and found agglutinating antibodies in 48% of those whose partners became pregnant and in 94% of those whose partners did not ( P < .01). The investigators did not believe, however, autoagglutination to be the mechanism for immunologic infertility in functional reversal failures because almost half of fertile men had the antibodies, autoagglutination of sperm was not seen in any of the study patients, and previous evidence had demonstrated in a rabbit model that immunologically induced sperm agglutination is neither necessary nor sufficient for sperm deactivation. In contrast, Requeda and colleagues concluded that sperm antibodies are an important cause of infertility in men who have undergone reversal surgery. In their study, six of eight fertile reversal patients had low titers of serum agglutinins, normal fertilizing capacity of their sperm, and no immobilizing antibodies, whereas six of seven infertile reversal patients had elevated serum agglutinins and four had agglutinating antibodies in seminal plasma and serum immobilizing antibodies.
Anatomic Failure: Sperm Granuloma
The effect of sperm granulomas on reversal surgery outcomes also has been evaluated. Working with a dog model, Schmidt determined that causes of vasovasostomy failure included sperm granuloma formation resulting from anastomotic leakage of sperm secondary to inadequate approximation of the vasal ends, puncture of the vasal lumen, misalignment of the anastomosis, and infection. Hagan and Coffey performed 119 vasovasostomies on 95 rats and found that sperm granulomas were present in 99% of the 49 failed anastomoses. They also performed vasal anastomoses in immature animals or in adult animals with suppression of spermatogenesis with testosterone and achieved 95% patency without granulomas. Alexander and Schmidt reported that more men who had sperm granulomas had sperm-immobilizing antibodies.
A healthy, patent epididymis is the cornerstone of successful vasectomy reversal surgery. Silber microsurgically explored the epididymis of 28 men undergoing vasectomy reversal who were found to have no sperm in the vasal fluid of the testicular side of the vas deferens. Sperm was found in 33 of 39 epididymides and histologic evaluation distal to the area with sperm revealed extensive interstitial sperm granulomas resulting from epididymal duct rupture. He concluded that persistent azoospermia after vasovasostomy resulted from secondary epididymal obstruction due to epididymal duct rupture from increased pressure after vasectomy.
Functional and technical problems do not account for all reversal failures. Continued infertility after surgery may be unrelated to surgical technique. For example, the epididymis also is important because of its role in sperm maturation. Schoysman and Bedford reported a greater chance of pregnancy after vasoepididymostomy with anastomosis to the corpus instead of the caput, with motility the only sperm characteristic affected. In most men whose anastomosis was located 8 mm or less from the proximal border of the caput, sperm were immotile. In contrast, 20% to 90% of sperm were progressively motile in cases where the anastomosis were greater than 10 mm from the caput border.
Macrosurgical technique
A wide range of macrosurgical techniques for vasovasostomy has been reported. The main variation in technique seems to be the use or omission of loupe magnification and stenting. Stenting is the use of a suture, tube, or other foreign body to help keep the lumen of the vas deferens open after a reversal procedure. Stents usually but not always are removed during the postoperative period.
Amelar and Dubin favored a nonstented technique with 4× loupe magnification using eight 6-0 Prolene sutures. Once the vas is exposed and the scarred ends are excised, the distal vas is cannulated with a blunt needle and tested for patency by injecting hydrogen peroxide dyed with methylene blue. Fluid from the proximal vas is examined with microscopy for the presence of sperm. These investigators used a stent to assist during the anastomosis but did not leave the stent in place to aid in healing. A 2-0 nylon suture is used as a stent during the creation of the anastomosis with the eight 6-0 Prolene sutures and is always removed before closure. This procedure is similar to the Schmidt operation, from which many of the derivative techniques have been adapted.
In contrast, Dorsey described a stented technique without magnification. He used a blunt 20-gauge needle with obturator to pierce the wall of the proximal vas, 1 centimeter from the site of anastomosis and the fascia, dartos, and scrotal skin. A zero monofilament dermalon suture is fed through the needle and the other end of the suture is advanced 12 to 14 cm into the distal vas segment. The anastomosis is formed over the stenting suture with four or five 6-0 Ethiflex sutures. The dartos layer is closed with a running 3-0 chromic suture. The stenting suture is exteriorized through the scrotal skin and is threaded through two lead shots, and the shots are crimped to keep the suture in place. The stenting suture is removed in 12 to 14 days, based on Schmidt’s finding that 7 to 10 days are required for epithelialization of the anastomosis. Dorsey reported successful reversal of vasectomy in 88% of 129 patients undergoing this procedure, including one patient who was 19 years post vasectomy.
Fitzpatrick reported the flap technique in 1978, in which both vasal ends are bivalved to create flaps. These flaps permanently widen the opening into each vasal lumen, resulting in a ballooning of the lumen at the site of anastomosis. In his preliminary study of 14 men, all had sperm in their ejaculate by postoperative week 6, and a 50% pregnancy rate was achieved by 3 months. Singh and Sharma used the Fitzpatrick flap operation with 2.5× magnification in 40 men and achieved patency and pregnancy rates of 79% and 34%, respectively. They proposed this spatulation technique as an alternative to the microsurgical approach for those without microsurgical training.
To stent or not to stent
Although “stent” and “splint” have been used interchangeably in the literature to describe the use of a foreign material to encourage patency of the vasal lumen during the time of epithelialization of the anastomosis, Montie and colleagues pointed out that a splint refers to something placed outside a structure to stabilize it whereas a stent is a compound for holding some form of graft in place. Stent, therefore, is the more accurate descriptor in this context. The use of exteriorized stents has several potential disadvantages, including infection and sperm leakage. Stents can provide a portal of entry for bacteria and serve as a foreign body to perpetuate the infection. Montie and colleagues tested in a canine model the feasibility of a completely intravasal stent using absorbable suture that does not require postoperative removal. Nineteen dogs underwent vasovasostomies with and without intravasal stents. The group without intravasal stents had a patency rate of 50% compared to 60% patency in the Dexon intravasal stent group and 70% patency in the chromic intravasal stent group. Rowland and colleagues confirmed these findings in 35 patients, with 86% patency using 3-0 plain catgut internal stents compared to 67% patency with externalized silkworm gut stents. The investigators pointed out, however, that patency rates varied widely by series: 60% to 67% for externalized gut stents, 83% to 95% for externalized nylon stents, 78% for intraoperative stainless steel stents, 92% for Silastic tube stents, and 100% for no stenting.
Using O’Conor’s 1948 questionnaire study as a model, Derrick and colleagues sent a survey to every member of the American Urological Association (2775 questionnaires). Of the 1363 replies, 542 urologists (40%) had performed at least one reversal procedure. The overall patency and pregnancy rates were 38% and 19.5%, respectively. This survey addressed the impact of stents on reconstructive success. The pregnancy rate for nonstented reversals was 10.9% compared to 19.9% to 26% for stented procedures (depending on the stent used).
The use of stents was the preferred method between 1940 and 1975. The disadvantages of stents were reported, including Fernandes and colleagues’ demonstration in dogs that obstruction often occurred at the site of exit of the stent through the vas deferens instead of at the anastomosis. As surgical technique improved with the widespread availability of microsurgery, stents gradually disappeared from reversal surgery.
Microsurgical technique
Owen and Silber, working independently, are credited with the development of the microsurgical vasovasostomy technique for clinical use. The use of the microscope for the anastomosis of the vas deferens in animals had been previously evaluated by several groups. The earliest reference to microsurgical vasovasostomy in humans was by Silber in 1975. Most of the initial animal studies involved a one-layer anastomosis, but Silber determined that in humans a two-layer is preferable largely because of the discrepant luminal diameters due to dilatation of the proximal vas segment. Silber performed the anastomosis under 16× to 25× magnification using single-armed 9-0 nylon sutures ( Fig. 3 ). He dilated the abdominal portion of the vas deferens with the insertion of jeweler’s forceps. The mucosal sutures included the elastic layer subjacent to the mucosa. After placement and tying of the three anterior sutures, the clamp is flipped to visualize the posterior wall of the anastomosis. He advocated careful inspection for gaps, tears, or inaccuracy of the lineup after placement of the six or seven mucosal sutures. The outer muscularis layer is then sutured separately. The two-layer technique allows for superior mucosal approximation and leakproof closure. Silber and colleagues performed histologic and electron microscopic studies and found that stricture was a common cause of failure with conventional or nonmicroscopic vasovasostomies and that obstruction of the vas deferens inhibited spermatogenesis. Further refinements to this microsurgical technique have been made over the years, including Goldstein’s introduction of the microspike approximator clamp ( Fig. 4 ) and use of microdots for precision suture placement. By providing a blueprint for the anastomosis, the dots minimize gaps and distortions of the anastomosis even with discrepant sizes of the cut vasal ends ( Fig. 5 ).
In 2004, Silber and Grotjan summarized their experience with 4010 cases of microscopic vasectomy reversal. Of 1357 patients undergoing microsurgical vasovasostomy, patency was achieved in 94.4%. Of 1008 patients undergoing unilateral vasoepididymostomy (with contralateral vasovasostomy) and 1013 patients undergoing bilateral vasoepididymostomy, patency rates were 93.7% and 78.7%, respectively.
In 1980, Lee and McLoughlin reported their comparison of macroscopic and microscopic vasovasostomy techniques. The macroscopic anastomoses were performed with a nonabsorbable monofilament internal stent, which was removed 7 to 14 days postoperatively and four to six absorbable sutures sized 4-0 to 6-0. The microsurgical anastomoses were performed in two layers using 8-0 to 10-0 synthetic suture. For the 61 patients undergoing the single-layer macroscopic procedure, patency and pregnancy rates were 90% and 46%, respectively. For the 26 patients undergoing the two-layer microscopic procedure, the rates were 96% and 54%, respectively. The investigators reported 100% patency and an 88% pregnancy rate in patients undergoing reversal surgery less than 2 years after the initial vasectomy and postulated importance of the 2-year period. Similarly, Silber had previously reported in 1977 the importance of duration of obstruction, with improved patency rates in those undergoing reversal surgery less than 10 years from the original vasectomy. Although opinion of the precise time interval between initial vasectomy and subsequent reversal has since changed, Lee and McLoughlin recognized the significance of the time interval to reversal outcomes. In 2004, Boorjian and colleagues reassessed obstructive intervals and determined that the pregnancy rate was significantly lower in those whose vasectomy had been performed more than 15 years before reversal surgery. Pregnancy rates were 89%, 82%, and 86% for obstructive intervals of 0 to 5, 5 to 10 and 10 to 15 years, respectively, compared to 44% for obstructive intervals greater than 15 years. Furthermore, Fuchs and Burt reported that spousal age is an important predictive factor after vasectomy reversal when surgery is performed 15 years or more after vasectomy.
The impact of the obstructive interval has been addressed by another important series published by the Vasovasostomy Study Group in 1991. The Vasovasostomy Study Group was a consortium of five institutions that pooled data for a 9-year period for a total of 1469 microsurgical vasectomy reversal procedures. Of the 1012 men who underwent reversal surgery for the first time and presented for postoperative semen analysis, 865 (86%) achieved patency. Four hundred twenty-one (52%) of 810 couples achieved pregnancy. The patency and pregnancy rates declined as the interval between the vasectomy and the reversal surgery increased: 97% and 76%, respectively, for interval less than 3 years, compared to 71% and 30%, respectively, for intervals greater than 15 years.
In 2004, Crain and colleagues published another questionnaire study on the practice patterns of vasectomy reversal surgery among practicing urologists and highlighted the significance of obstructive intervals. They received 622 completed questionnaires from 1508 mailed. Of the 59% who performed vasectomy reversals, 8% were fellowship trained in infertility, 23% were affiliated with residency training, and 69% practiced in a community setting, with fellowship-trained urologists performing more reversal procedures per year than the others. Fellowship-trained urologists also were more likely to perform surgery on patients greater than 15 years since vasectomy. Compared with the other two groups, they also were more likely to use the operating microscope (93% versus 65% and 56%) and examine the vasal fluid (83% versus 75% and 67%) and to use finer suture material.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree






