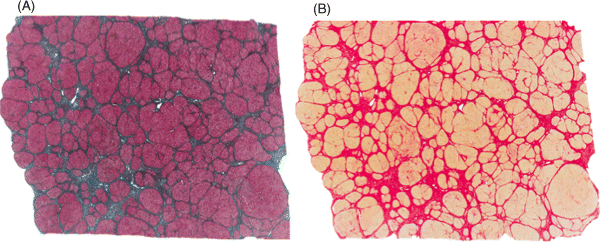
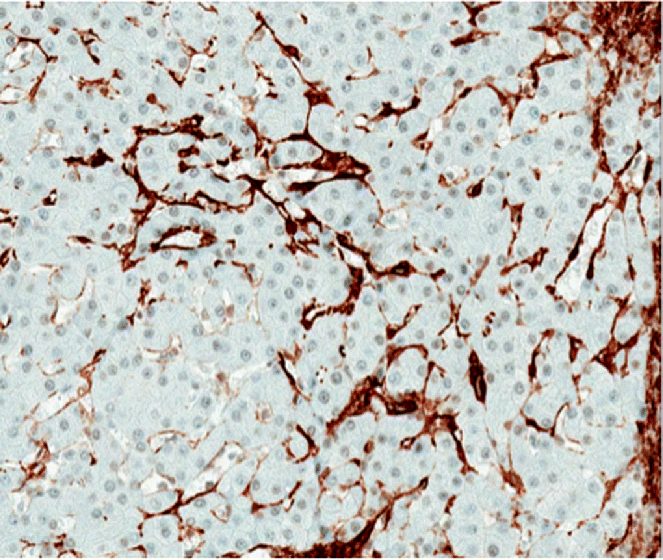
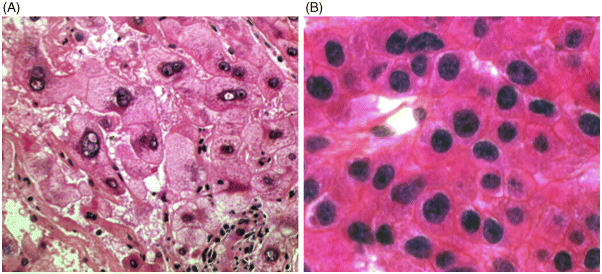
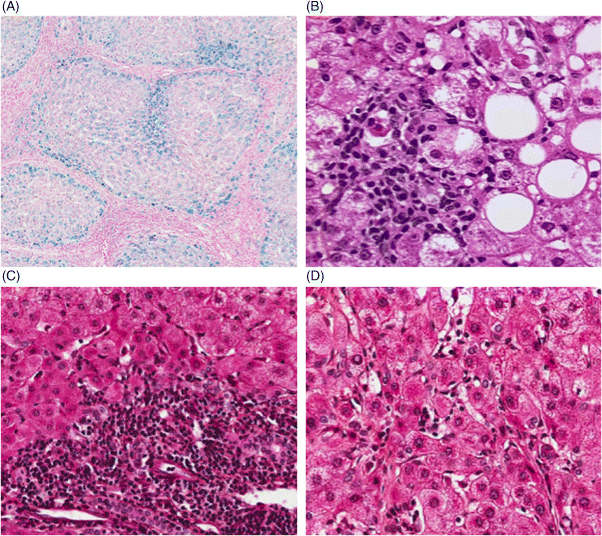
Chapter 4 Valérie Paradis Pathology Department, Beaujon Hospital, INSERM URM 1149 Paris France Usually considered as the end stage of chronic liver diseases (CLD), cirrhosis is defined anatomically by the presence throughout the liver of fibrous septa that delineate parenchymal nodules of various sizes. Such parenchymal changes may favor the occurrence of clinical or pathological complications, such as liver decompensation, portal hypertension, and development of primary liver malignancies, especially hepatocellular carcinomas (HCC). Although cirrhosis has been traditionally viewed as the end stage in the evolution of CLD, increasing evidence, with the successful treatment of several risk factors, especially related to viral infections, has supported the concept of reversibility of fibrosis and cirrhosis. The cirrhotic process is rather complex, involving necro-inflammatory lesions, production and/or accumulation of extracellular matrix (ECM), and vascular reorganization. Such a morphologic picture may be appreciated best by the histologic approach which also provides, when necessary, clues for etiologic factors. Cirrhosis is defined by a diffuse process characterized by fibrosis and the conversion of normal liver architecture into structurally abnormal nodules [1]. Diagnosis of cirrhosis relies on the presence of extensive fibrosis delineating parenchymal hepatocellular nodules associated with vascular abnormalities reflecting the occurrence of intrahepatic vascular shunts [2]. Intrahepatic accumulation of ECM is observed at various sites of the parenchyma, mainly concerning the perisinusoidal space of Disse (defining the space between the vascular channel and the hepatocytes) and portal tracts. ECM deposits increase with the degree of severity of fibrosis, moving from less than 3% of the relative area in a normal liver to more than 15% in a cirrhotic liver [3–5]. In addition to such quantitative aspects, significant qualitative modifications of the ECM components are observed with prominent production of fibrillar collagens contributing to sinusoidal capillarization, a phenomenon that strongly impairs exchanges between liver cells and blood flow [6]. On liver tissue specimens, excess deposits of ECM are easily observed by specific staining (trichrome, picrosirus). Additional procedures, such as histochemistry and immunohistochemistry, are potential tools to define the main types of deposited matrix proteins (Figure 4.1). Second harmonic generation (SHG) microscopy offers the opportunity to image fibrillar collagens in unstained tissues. As liver fibrosis is mainly associated with deposition of type I and III collagens, such an approach, based on multiphoton microscopy, may be used as a collagen scoring method in CLD [7]. Interestingly, its performance has been demonstrated in a cohort study of patients with CLD related to viral hepatitis, showing further discrimination of several levels of fibrosis as assessed by semi-quantitative staging systems [8]. Figure 4.1 Cirrhosis at low power view. Specific stainings of fibrosis showing parenchymal nodules delineated by annular fibrous septa: (A) trichrome staining; (B) picrosirius staining. Production of ECM results from the activation and proliferation of fibrocompetent cells, which are able to produce and excrete the main ECM components. They include hepatic stellate cells (HSC), which correspond to fat-storing cells located in the perisinusoidal space, and portal fibroblasts [9,10]. Although other cell types of the liver, such as biliary cells, are able to produce ECM components they represent a minor source of liver fibrogenesis [11]. The role of HSC has been investigated in this process where they acquire an activated phenotype of myofibroblast requiring crosstalk with mostly inflammatory cells and production of a wide range of cytokines and chemokines, such as transforming growth factor β1 (TGFβ1), connective tissue growth factor (CTGF), and so on [12,13]. Interestingly, presence of activated HSC on liver tissue sections may be identified using specific immunophenotypical markers, such as α smooth muscle actin (Figure 4.2). Such a procedure gains further insights into the dynamics of liver fibrogenesis and may be valuable in the prediction of the course of CLD, as already illustrated in the follow-up of patients transplanted for hepatitis C virus (HCV) cirrhosis [14]. The fibrous compartment is not restricted to an amorphous area of ECM components but commonly houses different cell types in addition to fibroblasts. Among these, inflammatory cells and biliary proliferation are the most prominent, even though their respective proportions may vary considerably according to the origin of the cirrhosis and its severity. Figure 4.2 Hepatic stellate cells in cirrhosis. α-Smooth muscle actin immunostaining showing hepatic stellate cells in parenchymal nodules, located in peri-sinusoidal space (Disse space). Different morphologic patterns of fibrosis are recognized according to their origin and severity [15]. Thus, deposition of ECM usually starts in portal tracts and periportal areas in different contexts, including chronic viral hepatitis, autoimmune and biliary diseases, while an earlier involvement of the centrolobular zone is observed in alcoholic and nonalcoholic steatohepatitis or vein outflow disorders. Later on, when fibrosis progresses, the fibrous septa connect vascular structures to each other, leading to constitution of portal to portal, portal to central septa, or central to central septa, mainly depending on the etiologic risk factors and severity of the disease. However, at the advanced stage, all kinds of extensive septa are observed throughout the liver, hampering the recognition of the elementary anatomic structures of the liver (i.e., portal tracts and centrolobular veins). Altogether, this architectural distortion leads to a macroscopic picture of cirrhosis commonly defined by the global aspect of the liver (hypertrophic, atrophic, dysmorphic) and size of the hepatocellular nodules into macro- or micronodular according to whether the majority of the nodules are larger or smaller than 3 mm. Macronodular cirrhosis, especially observed in hepatitis B virus (HBV) infection, may be composed of nodules of several centimeters in diameter. In fact, in a significant number of cases, cirrhosis is made up of a mixture of small and large parenchymal nodules, recognized as “mixed” or “irregular” cirrhosis. Importantly, in the course of cirrhosis, the size of the nodules may evolve, with progression of micronodules to macronodules in cases of successful removal of the etiologic agent. In addition to the fibrogenic process, vascular structural changes within the intrahepatic circulation are essential to the increased vascular resistance and physiopathology of portal hypertension [16,17]. Importantly, these modifications directly correlate with the degree of fibrosis [18,19]: (i) angiogenesis, driven by hypoxia, mainly through the production of vascular endothelial growth factor (VEGF), leading to increase in vascular structures with additional shunting, and (ii) sinusoidal remodeling featured by increased density of contractile HSC around sinusoidal endothelial cells that display significant morphologic and functional changes, with the loss of their characteristic fenestra and the occurrence of a basement membrane, known as sinusoidal capillarization, resulting in sinusoidal vasoconstriction [16,17]. Such features can be seen on tissue sections showing increased number of vessels in the fibrous septa, perisinusoidal fibrosis into the nodular parenchyma, and CD34 immunostaining of the sinusoidal lining (Figure 4.3). Figure 4.3 Vascular changes in cirrhosis. (A) Low magnification showing cirrhosis with vessels multiplication in fibrous septa (hematein and eosin). (B) Higher magification of cirrhotic nodule with sinusoidal dilatation. (C) CD31 immunostaining dysplaying increased vascular density. (D) Sinusoidal capillarization demonstrated by CD34 lining of endothelial cells in cirrhotic nodule. Besides the architectural modifications observed in cirrhosis, a wide range of elementary histologic features may be present. Among them, significant disarray of hepatocellular trabeculae is observed, with focal thickening of liver cell plates to two or three cells thick, consistent with regenerative process. Silver stainings for reticulin clearly demonstrate these changes with an irregular and focally thickened pattern. Cytologically, hepatocytes may appear regular, as eosinophilic polygonal cells, and may harbor dystrophic changes (irregularity in shape and size), or dysplastic changes. These latter modifications, now described as small or large liver cell changes, usually involve hepatocellular clusters, only recognized at the microscopic level (Figure 4.4). Figure 4.4 Dysplastic changes in cirrhosis. (A) Large cell change represented by large cells containing atypical nuclei of increased size. (B) Small cell change defined by foci of small hepatocytes with increased nuclear: cytoplasmic ratio (hematein and eosin). Large cell change (LCC), first described in chonic hepatitis B, is defined by large cells containing atypical nuclei of increased size, sometimes multinucleated, but with a fairly normal nuclear: cytoplasmic ratio [20]. By contrast, small cell change (SCC) consists of small hepatocytes containing irregular and hyperchromatic nuclei with increased nuclear: cytoplasmic ratio [21,22]. Iron overload is commonly present in parenchymal nodules, especially in patients with advanced cirrhosis, whatever its etiology [23]. Its intensity is variable, well-demonstrated with Perls’ staining, usually heterogeneous from one nodule to another, involving both macrophages and hepatocytes (Figure 4.5). Several semi-quantitative scoring systems have been developed, based on the quantity and/or the natural progression of accumulation (from periportal to centrolobular zones) [24]. In cases of hereditary hemochromatosis, a pericanalicular pattern of iron distribution is described in the hepatocytes. However, with progression of the disease and extension of fibrosis, there is loss of the characteristic gradient pattern (portal centrolobular zone) and iron overload is also seen in the biliary epithelium. Features of steatosis and steatohepatitis may also be observed in cirrhotic samples, more commonly associated with metabolic syndrome (MS) and chronic alcohol consumption [25–28]. In those settings, steatosis is prominently macrovacuolar, and, interestingly, the degree of steatosis seems to decrease with extension of fibrosis. Such an observation may explain the increased prevalence of MS and its risk factors, such as obesity and diabetes, in patients with so-called cryptogenic cirrhosis [29]. Morphologic features of steatohepatitis include clarified and ballooned hepatocytes that may contain Mallory–Denk bodies potentially associated with inflamatory cells (mostly lymphocytes in MS and neutrophil polymorphs in alcoholic diseases) (Figure 4.5). Figure 4.5 Parenchymal nodules: elementary features. (A) Iron overload in parenchymal nodules involving mainly hepatocytes with a gradient pattern (Perls’ staining). (B) Steatohepatitis with ballooned hepatocytes containing Mallory–Denk bodies with inflamatory cells around (mostly lymphocytes in metabolic syndrome and neutrophil polymorphs in alcoholic diseases) (hematein and eosin). (C) Piecemeal necrosis: portal inflammation with inflammatory cells disrupting limiting plate (hematein and eosin). (D) Lobular inflammation composed of small foci of lymphocytes between hepatic cell plates (hematein and eosin).
Histology/Pathology
Introduction
Cirrhosis: A Pathologic Spectrum
Fibrosis: Extracellular Accumulation of Matrix Components
Vascular Changes

Parenchymal Nodules: Etiologic Features
Cirrhosis: An Evolving Concept
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree







