Gastric cancer is the third common cancer and is the second leading cause of cancer deaths worldwide. Endoscopy is being increasingly used for gastric cancer screening because of a high detection rate. Despite promising data, the technique depends heavily on the availability of endoscopic instruments and expertise for mass screening. Furthermore, the introduction of various new endoscopic devices and techniques may enhance the value of endoscopy in efficacious cancer screening. High-definition endoscopy and image-enhanced endoscopy, including narrow band imaging, are the key modalities in advanced endoscopic imaging in gastric cancer.
Gastric cancer is the third common cancer and is the second leading cause of cancer deaths worldwide. Because the high mortality from gastric cancer mainly results from late presentation in advanced stage of cancer with metastasis, efficacious screening and curative treatment at an early stage is one of the main ways to reduce gastric cancer death. Endoscopy is being increasingly used for gastric cancer screening because of a high detection rate. Despite promising data, the technique depends heavily on the availability of endoscopic instruments and expertise for mass screening. Therefore, it is key for the efficacious early detection of gastric cancer to perform endoscopy in the high-risk population selected by a promising nonendoscopic biomarker, such as serum pepsinogen status. Furthermore, the introduction of various new endoscopic devices and techniques may enhance the value of endoscopy in efficacious cancer screening. High-definition endoscopy (HDE) and image-enhanced endoscopy, including narrow band imaging (NBI), are the key modalities in advanced endoscopic imaging in gastric cancer.
HDE improves the detection and diagnosis of superficial gastric cancer
Although upper gastrointestinal (GI) endoscopy is more accurate than nonendoscopic modalities, including integrated positron emission tomography and barium meal examination, it is empirically known that considerable numbers of superficial gastric cancers are missed or misdiagnosed with conventional white light endoscopy (WLE). HDE has been developed for improving the image quality and diagnostic accuracy of WLE. To elucidate the potential of HDE, we prospectively compared ultrathin endoscopy (UTE) with HDE with respect to diagnostic accuracy of superficial gastric neoplasia.
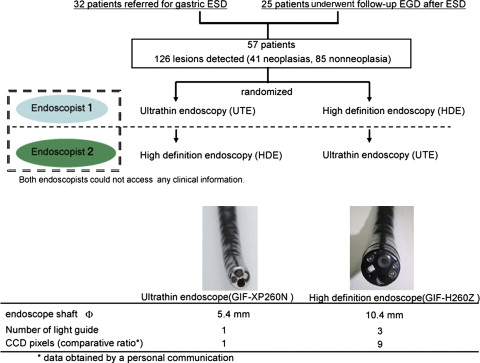
In this study, 32 patients with superficial gastric neoplasia referred for endoscopic submucosal dissection (ESD) were recruited; 25 patients undergoing follow-up endoscopy after ESD were also enrolled as neoplasia-free controls ( Fig. 1 ). Patients with obvious advanced gastric carcinomas or cancerous lesions with deep invasion to the gastric submucosa were excluded. Each patient underwent UTE (GIF-XP260N, Olympus Medical Systems Corp, Tokyo, Japan) and HDE (GIF-H260Z, Olympus Medical Systems Corp) back-to-back in a randomized order. UTE and HDE were independently performed by 2 different experienced endoscopists who did not have access to any clinical information before the endoscopic examination. The study coordinator organized the patient data and guided the 2 endoscopists, one at a time, into the endoscopic room. The endoscopists independently performed endoscopic examination under conscious sedation on the same patient and recorded the diagnosis of neoplastic as well as nonneoplastic lesions, including gastric ulcers or gastric polyps. All lesions recorded as neoplastic or nonneoplastic, as well as any neoplastic lesion that was referred for ESD but not detected by either endoscopist, were biopsied by the second endoscopist under the supervision of the study coordinator, who attended all endoscopic examinations and was aware of the diagnosis made by the 2 endoscopists. The study coordinator could easily notice the existences of gastric neoplasias referred for ESD but missed by the participating endoscopists because he was aware of the pathologic reports and representative endoscopic images recorded at the affiliated hospitals. The pathology results from the biopsy were used as a gold standard for the diagnosis of gastric cancer.
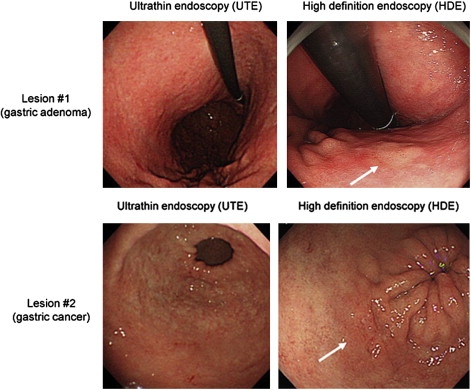
Among 57 enrolled patients, 41 lesions (16.5 ± 13.5 mm in diameter, mean ± SD) were pathologically diagnosed as neoplasias (27 carcinomas and 14 adenomas). Of the 41 pathologically diagnosed neoplasias, 11 were not detected and 3 were diagnosed as nonneoplasias by UTE, indicating that the missing and misdiagnosis rates of UTE were 26.8% and 14.6%, respectively. Similarly, 5 of the 41 pathologically diagnosed neoplasias were not detected and 4 were diagnosed as nonneoplasias by HDE, indicating that the missing and misdiagnosis rates of HDE were 12.2% and 9.8%, respectively. The rate of missed and misdiagnosed proximal lesions (fornix and corpus) was significantly higher for UTE (29%) than for HDE (7.2%) ( P = .002). In comparison, the rates of missed lesions and misdiagnosed distal lesions (angulus and antrum) were comparable for UTE and HDE ( Table 1 ). Representative neoplastic lesions missed by UTE but correctly diagnosed by HDE are shown in Fig. 2 .
| (A) Comparison of the diagnostic accuracy in the diagnosis of gastric cancer | ||||
|---|---|---|---|---|
| Sensitivity | Specificity | |||
| UTE | 58.5% | P = 0.021 | 91.8% | P = 0.014 |
| HDE | 78.0% | 100% | ||
| (B) Rates of missed and misdiagnosed lesions including neoplasia and nonneoplasia | ||||||
|---|---|---|---|---|---|---|
| All Lesions (95% CI a ) | Distal Lesions (Angulus-Antrum) | Proximal Lesions (Fornix-Corpus) | ||||
| UTE | 27.1% (18.5–38.3%) | P <0.001 | 24.5% (13.3–38.9%) | P = 0.19 | 29.0% (18.7–41.1%) | P = 0.002 |
| HDE | 9.3% (4.7–16.7%) | 12.2% (4.6–24.7%) | 7.2% (2.4–16.1%) | |||
Although conscious sedation is widely used to reduce endoscopy-induced discomfort and increase acceptance of the procedure, it can cause adverse effects and increases the cost, either directly (medications, additional time required to sedate and recover the patients) or indirectly (time lost from work by both the patient and the patient’s escort). UTE has emerged as an alternative to sedated conventional upper GI endoscopy for screening because it is well tolerated in the absence of sedation and is less costly. However, the authors’ study demonstrated that the diagnostic accuracy of HDE is significantly higher than that of UTE for superficial gastric neoplasia and that this difference is particularly striking for neoplasias in the proximal stomach. We need to adequately select HDE or UTE in different clinical settings by recognizing the differences in the diagnostic accuracy and the acceptance without sedation.
The clinical value of autofluorescence endoscopy is limited in gastric cancer
Although HDE improves the diagnostic accuracy of WLE, more than 20% of superficial gastric neoplasias are still missed or misdiagnosed. Image-enhanced endoscopy, including autofluorescence endoscopy (AFE) and NBI, has been developed for overcoming the limit of WLE. There has been considerable interest in the use of AFE for the detection of early neoplasias, and the diagnostic relevance of the modality has been demonstrated for the detection of early neoplasias in the esophagus and colon.
Therefore, the authors conducted a prospective study to systematically compare AFE with WLE for the detection of superficial gastric neoplasia. The authors enrolled an enriched population of 33 patients with superficial gastric neoplasia referred for ESD and 18 patients undergoing follow-up endoscopy after curative ESD as control. An autofluorescence imaging system (CLV-260SL and CV-260 SL, Olympus Medical Systems Corp) and the autofluorescence imaging scope (XGIF-Q240FZ, Olympus Medical Systems) were used for the AFE procedure. The new autofluorescence system enables selection of either a red-green-blue illumination for normal WLE or an excitation/reflected light combination for AFE. At the direction of a study coordinator, 2 endoscopists blinded to the patient’s history and to each other’s findings, performed WLE followed by AFE or AFE alone, in a random order. Both endoscopists independently recorded the presence of lesions observed by AFE and WLE. All lesions identified in either test were biopsied, and the pathologic results were used as the gold standard.
A total of 39 gastric neoplasias were histologically confirmed, and 52 pathologically diagnosed nonneoplastic lesions were found to be either WLE- and/or AFE-positive. Sensitivities of WLE and AFE alone were 74% and 64% ( P = .79), respectively, and specificities of WLE and AFE alone were 83% and 40% ( P = .0003), respectively. WLE followed by AFE had a sensitivity of 69% (nonsignificant) and a specificity of 64% ( P = .046) compared with WLE alone. AFE detected 13% of all neoplasias finally diagnosed (23% of elevated neoplasias), which were missed by WLE.
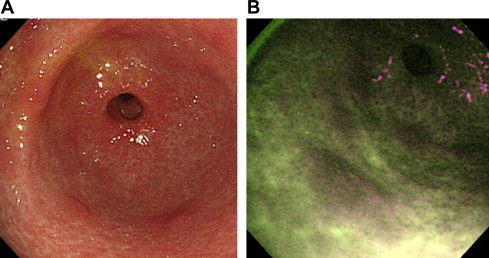
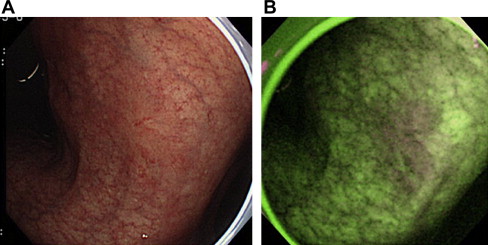
Although one-quarter of elevated gastric neoplasias were detected only by AFE, it was concluded that its specificity is poor because of the high rate of false-positive detection of lesions, such as intestinal metaplasia or regenerative hyperplasia; therefore the clinical value of AFE is limited. Fig. 3 shows false-positive detection of lesions by AFE, and Fig. 4 demonstrates a representative gastric cancer that was missed by WLE but detected by AFE in the prospective study.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree








