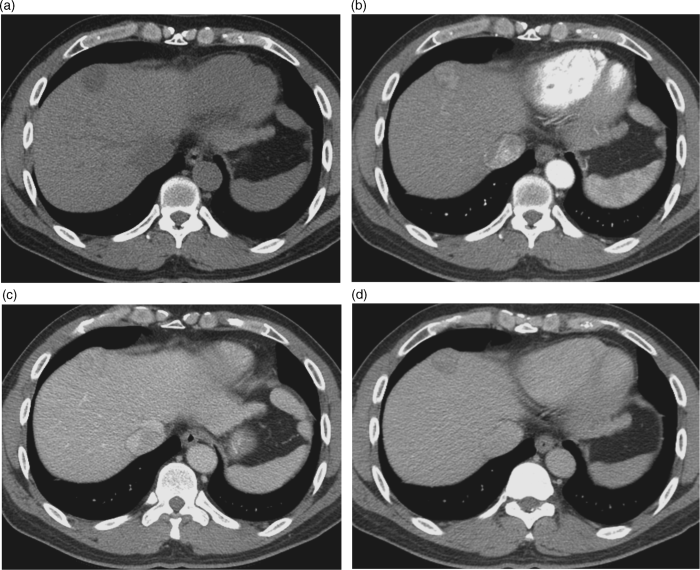
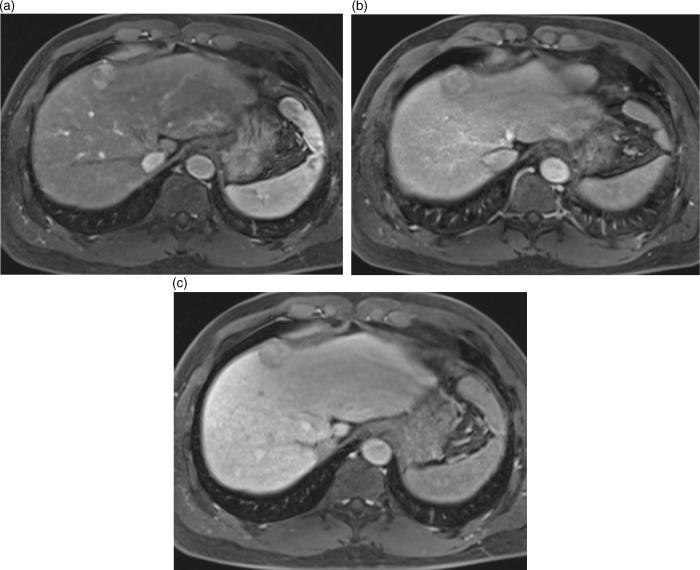
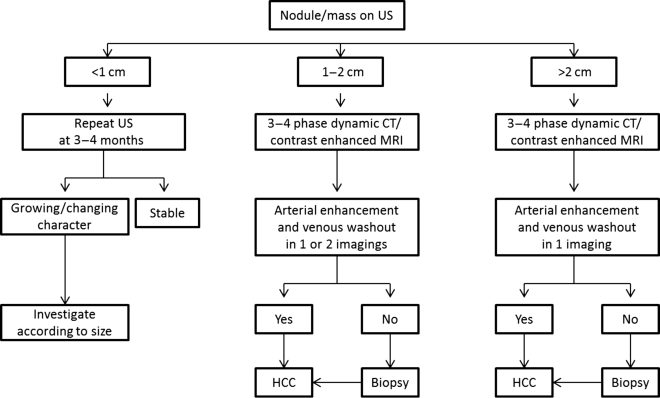
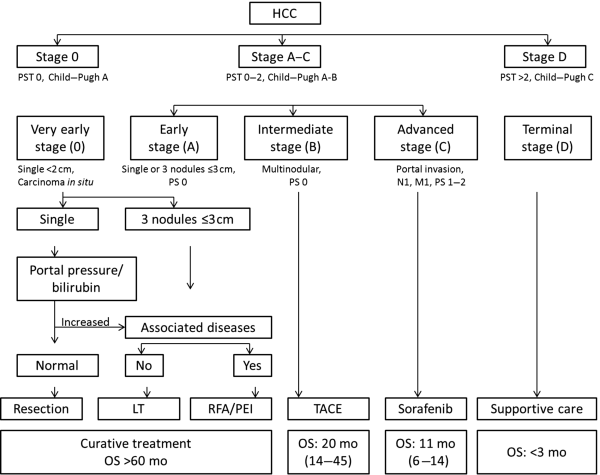
Chapter 10 Kwang-Hyub Han and Do Young Kim Department of Internal Medicine, Yonsei University College of Medicine Seoul Korea Hepatocellular carcinoma (HCC) accounts for 70–85% of the total primary liver cancers worldwide [1]. HCC is diagnosed in more than a half million people annually and is a fatal disease, the second most frequent cause of cancer-related deaths in males [2]. The global distribution of HCC prevalence represents a difference in etiologic factors of HCC among geographic areas. The highest prevalence of HCC in Eastern Asia and sub-Saharan Africa primarily comes from chronic hepatitis B virus (HBV) infection in these areas. Hepatitis C virus (HCV) infection is also a major cause of HCC development in the United States, Japan, and central European countries [3]. Depending on the region, there are other risk factors including alcohol, obesity, and nonalcoholic steatohepatitis (NASH), α-1 antitrypsin deficiency, hemochromatosis, autoimmune hepatitis, and Wilson’s disease [4]. Cirrhosis is recognized as the most important risk factor of HCC, and the annual risk of HCC development is 3–8% in HBV-associated cirrhosis and 1–7% in HCV-related cirrhosis [4,5]. In addition, the probability of HCC development is directly correlated with the stage of liver fibrosis in patients with chronic liver disease [6]. The symptoms caused by HCC are variable, nonspecific, and hard to differentiate from complications of cirrhosis per se. Although fatigue and loss of appetite might be the initial symptoms, patients with early stage HCC usually do not present with any symptoms. Abdominal pain is uncommon, but when present usually signifies a very large tumor extending to the surface of liver capsule or extrahepatic spread. Weight loss and unexplained fever are alarming signs that should trigger a search for HCC in cirrhotic patients. Deterioration of hepatic function in cirrhotic patients, from compensated to decompensated state, or aggravation of cirrhotic complications can be accompanied by HCC development. HCC invasion to the portal vein may decrease blood flow into the liver, which in turn leads to jaundice, ascites, edema, hepatic encephalopathy, or variceal hemorrhage. When HCC grows and metastasizes to other organs, varied symptoms of new onset can occur, such as bone pain (bony metastasis), dyspnea (lung metastasis), and neurologic symptoms (brain or spinal metastasis). On physical examination, a palpable mass with or without tenderness on the right upper quadrant is often a finding to suggest that HCC has already progressed to an advanced stage. Jaundice is a bad prognostic sign, and implies HCC invasion into the portal vein or bile duct, or poor underlying liver function. Other signs including muscle wasting, pitting edema, ascites, and palmar erythema are not specific to HCC because they can also be observed as cirrhotic complications. Screening is a one-time application of tests for detecting a disease at an early stage. For HCC, the term “surveillance,” which is repeated application of screening, is more appropriate because patients with risk factors have life-long probability of developing HCC [7]. As surveillance has been found to significantly reduce HCC mortality by detecting more cancers at an early stage, it is recommended in high risk populations (i.e., cirrhotics with or without chronic HBV or HCV infections) [8]. Surveillance is usually performed using abdominal ultrasound (US) and serum alpha-fetoprotein (AFP) measurement at regular intervals [9]. However, US is more sensitive and specific than AFP elevation for early detection of HCC [10]. The incorporation of serologic markers in surveillance program to complement US is a matter of debate. Any tumor marker including serum AFP is not recommended as a surveillance test in Western countries because of its inadequate sensitivity and specificity [10–13]. On the contrary, data from clinical practice suggest that performance of AFP might be better than US and that combination of US and AFP could improve sensitivity to 90% with minimal loss of specificity [14]. The interval for HCC surveillance in cirrhotic patients has been set at 6–12 months, based on the estimated tumor growth rate. Six-month interval seems to be superior to 12 month in terms of detection of early stage HCC, application of curative treatment, and overall survival [15]. Unlike other solid tumors, the diagnosis of HCC is largely made by noninvasive methods such as computed tomography (CT) scan and magnetic resonance imaging (MRI). Although liver biopsy for pathologic confirmation has several limitations of false-targeting, risk of bleeding complication, and tumor seeding, it is currently the gold standard of HCC diagnosis [16]. However, the increasing accuracy and specificity of imaging studies has largely eliminated the need for routine liver biopsy (Figure 10.3). HCC usually takes a multi-step carcinogenesis from chronic inflammation to low or high grade dysplastic nodule (DN) and to small HCC. DN, a pre-cancerous lesion, is characterized by a distinctly nodular lesion (1 cm in size) differing from the surrounding liver parenchyma regarding size, color and texture, and bulges on the cut surface. The cellular atypia is at least moderate in high grade DN, compared with mild in low grade DN, but insufficient for HCC diagnosis. The differentiation between high grade DN and well-differentiated HCC is often difficult and identification of stromal invasion of tumor cells supports the HCC diagnosis [17]. Dynamic contrast-enhanced CT scan is an essential part of HCC diagnosis. Furthermore, the enhanced speed and flexibility of multi-detector CT (MDCT) has enabled higher spatial and temporal resolution of small lesions. Following rapid intravenous infusion of contrast, images are acquired at various time intervals corresponding to the phase of contrast enhancement. HCC derives blood flow predominantly from the hepatic artery and tends to enhance during the arterial phase or 2–40 seconds after contrast infusion. On the contrary, the surrounding hepatic parenchyma obtains 75–80% of its blood flow through the portal vein and is best demonstrated 50–90 seconds after contrast infusion during the portal phase. This “arterial enhancement” and “venous washout’” are a pattern typical of HCC on dynamic CT scan (Figure 10.1) [18]. MRI offers higher tissue contrast than CT in the evaluation of HCC. Moreover, gadoxetic acid, which behaves as an extracellular agent in the early phase and as a hepatobiliary agent in delayed phase, may allow for accurate diagnosis based on hemodynamics and hepatocyte function (Figure 10.2). MR scan using this contrast agent appears to have better performance than multi-phase CT scan in the differentiation of HCC from chronic liver disease, especially for a lesion <1 cm [19,20]. Figure 10.1 Four-phase, contrast-enhanced images of hepatocellular carcinoma from computed tomography (CT) scan. (a) Pre-contrast phase; (b) a hypervascular, enhancing nodule is observed in the anterior, superior right lobe of liver in the arterial phase; (c) the contrast has washed-out from the nodule in portal venous phase; (d) more wash-out is seen in delayed phase. Figure 10.2 Features of dynamic magnetic resonance imaging (MRI) of hepatocellular carcinoma. (a) An enhancing nodule-in-nodule is noticed in arterial phase; (b) wash-out of contrast dye from the nodule in portal venous phase; (c) the nodule shows low signal intensity compared with the neighboring parenchyma in delayed phase. Contrast-enhanced ultrasound (CEUS) is an imaging technique that uses microbubble contrast agents that are small (3–5 μm) enough to pass thorough the pulmonary circulation. After a bolus injection, the contrast agents show strong vascular enhancement and slowly diffuse into the blood over about 5 minutes [21]. In particular, Sonazoid, a new contrast agent, can be engulfed by Kupffer cells and has a postvascular phase which begins 10 minutes after contrast injection and lasts for 60 minutes. Lack of Kupffer cells in HCC shows an enhancement defect in postvascular phase while benign lesions show sustained enhancement [22]. The advantages of CEUS over dynamic CT or MRI are no deterioration of renal function, real-time assessment of enhancement pattern, and good tolerability [21]. Clinical criteria for noninvasive diagnosis of HCC has been evolving with the increasing accuracy of dynamic studies such as CT and MRI scans. In cirrhotic patients, a nodule or mass >2 cm can be diagnosed as HCC if the imaging finding is compatible with HCC (i.e., arterial hypervascularity and portal/venous washout), in one of multi-phase CT and MRI. A nodule between 1 and 2 cm poses a diagnostic challenge even by biopsy [23–25]. It remains controversial whether one or two dynamic imaging modalities are necessary for the lesion sized 1–2 cm. For a lesion less than 1 cm, repeated US follow-up with a 3–4 month interval is recommended to see any growth, because most of the lesions are large regenerative nodules or dysplastic nodules. The diagnostic algorithm for HCC is presented in Figure 10.3. The use of CEUS in HCC diagnosis has been excluded from updated American Association for the Study of Liver Diseases (AASLD) guidelines for the reason that intrahepatic cholangiocarcinoma can be misdiagnosed as HCC [26]. However, this issue needs to be further studied. Figure 10.3 Diagnostic algorithm of hepatocellular carcinoma (HCC) (modified from AASLD and EASL guidelines). CT, computed tomography; MRI, magnetic resonance imaging; US, ultrasonography. Adapted from AASLD guidelines (www.aasld.org/practiceguidelines) and EASL guidelines (www.easl.eu/_clinical-practice-guideline). It is important to assess the stage of HCC accurately and consistently at the time of diagnosis for stratifying patients to predict overall survival, facilitate treatment, and enable comparison of research outcomes. Because of the heterogeneity and complex clinicopathologic characteristics of HCC as well as cirrhotic background which is accompanied in most HCCs, various staging systems have been developed. The tumor–node–metastasis (TNM) staging system proposed by the American Joint Committee on Cancer (AJCC) is the most widely applied in solid cancers [27]. Although this staging was originally based on pathologic information after surgical operation, validation in HCC patients who underwent nonsurgical treatment has been tried according to advancement in imaging techniques [28]. As the overall survival of HCC patients is substantially affected by underlying liver function, several staging systems included Child–Pugh score or class. The Cancer of Liver Italian Program (CLIP) score combines tumor factors (tumor morphology, AFP, portal vein thrombosis) and Child–Pugh score to generate a simple scoring system [29]. Similarly, Japanese Integrated Staging (JIS) combines Japanese TNM system and Child–Pugh class to produce a score of 0–5 [30]. The prognostic value of these staging systems has been validated internally or externally [31,32]. However, it appears that none can be recommended universally because of the lack of validation in both Western and Asia-Pacific populations. The Barcelona Clinic Liver Cancer (BCLC) staging system, proposed in 1999, is the only one that is endorsed by AASLD and the European Association for the Study of the Liver (EASL) [33]. This is unique in that it offers specific treatment recommendations for each stage. Four categories of A (early), B (intermediate), C (advanced), and D (end-stage) are based on the tumor stage (tumor size, number of tumors, presence of portal vein invasion), liver function (Child–Pugh class, bilirubin level, portal hypertension), performance status indicated by European Cooperative Oncology Group (ECOG) scale, and cancer-related symptoms (Figure 10.4). BCLC B and C stages have broad ranges of tumors resulting in a wide range of survival, which makes it challenging for this staging system to provide a fine stratification of patients for clinical trial design. Refinement of intermediate and advanced stages combined with molecular classification like other cancers such as breast cancer would be more helpful. Figure 10.4 Barcelona Clinic Liver Cancer (BCLC) staging system. LT, liver transplantation; mo, month; OS, overall survival; PEI, percutaneous ethanol injection; PST, performance status; RFA, radiofrequency ablation; TACE, transarterial chemoembolization. Adapted from AASLD guidelines (www.aasld.org/practiceguidelines) and EASL guidelines (www.easl.eu/_clinical-practice-guideline).
Hepatocellular Carcinoma
Introduction
Clinical Features
Surveillance of HCC
Diagnosis and Staging
Treatment
Surgical Treatment
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree








