Hepatocellular Cancer: Anatomy and Staging
Christopher J. Gannon
Steven A. Curley
Introduction
Hepatocellular carcinoma (HCC) is a common malignancy worldwide, with more than 500,000 cases presenting annually (1). Although this prevalence is not mirrored in the population of the United States, the incidence of HCC continues to increase, with more than 19,000 estimated new cases expected in 2007 in the United States. Unfortunately, this disease remains highly lethal, with more than 17,000 deaths anticipated that same year (2). Chronic liver disease is the common underlying cause of HCC.
Worldwide, chronic viral hepatitis is primarily responsible for hepatic dysfunction and cirrhosis. In Western countries, other factors such as excessive, chronic alcohol consumption may have an impact on the carcinogenesis of HCC. Hepatitis B virus (HBV) is already a well-documented entity responsible for the underlying hepatic injury found in patients with HCC. Similarly, chronic infection with hepatitis C virus (HCV) has been shown to be a powerful etiologic element (3). Despite the international prevalence and increasing incidence within the United States, successful therapy is limited to patients with early stage disease and is typically dependent on clinical factors as well as the anatomy of the cancer. As a result, multiple staging systems for HCC have been developed. We discuss the systems that have gained wide acceptance through clinical verification.
Anatomy
A thorough understanding of the anatomy of the liver is necessary to assess patients as candidates for surgical resection or other local therapies. The vasculature of the liver has robust inflow with the hepatic artery, a branch of the celiac artery, and the portal vein, venous blood from the intestinal tract. Consistent nomenclature is also paramount to improve understanding among caregivers and to clarify the sometimes divergent literature.
The liver lies in the right upper quadrant of the abdomen, nestled underneath the rib cage. It is completely surrounded by a peritoneal membrane, known as Glisson capsule. This investing sheath also envelopes the portal vascular structures as they enter the liver. In contrast, hepatic veins are not covered by Glisson capsule. The liver receives a dual blood supply from both the hepatic artery and portal vein. The portal vein supplies approximately 75% of the blood supply, with the hepatic artery providing the remainder. The liver is drained predominantly by three major veins: the left, middle, and right hepatic veins. The remaining drainage occurs through several small veins that directly enter the vena cava from the posterior aspect of the liver.
For surgical approaches to hepatic tumors, it is important to recognize the variability of the extrahepatic arterial anatomy (4,5). In the majority of patients, the common hepatic artery arises from the celiac trunk giving off the gastroduodenal artery, followed by a right gastric artery. The proper hepatic artery gives rise to the cystic artery followed by the right and left hepatic arteries. However, the cystic artery frequently arises from the right hepatic artery and rarely may arise from the left hepatic artery. Anatomical variants include a replaced right hepatic artery (right hepatic artery arising off the superior mesenteric artery) and a replaced left hepatic artery (left hepatic artery arising from the left gastric artery). The importance of these variants in hepatic surgery is in recognizing their presence so as to prevent inadvertent injury. Other anatomical variants exist, including accessory vessels (a vessel that exists in conjunction with a vessel with a more standard origin) and variations in the cystic artery anatomy.
An understanding of biliary anatomy and its variations is essential to perform surgery for hepatic neoplasms successfully. The proximal right hepatic duct is largely intrahepatic, while the proximal left hepatic duct is extrahepatic and runs perpendicular to the common hepatic duct to the level of the round ligament. At the round ligament, the left hepatic duct is formed by confluence of ducts from segment IV and segments II/III. The confluence of the left and right hepatic ducts is cephalad and ventral to the portal vein bifurcation. The hepatic bile duct confluence gives rise to the common hepatic duct, the portion of the duct between the confluence and the cystic duct entrance. The common bile duct extends from the cystic duct to the ampulla of Vater. The blood supply to the extrahepatic bile duct arises from the right hepatic artery superiorly and from the gastroduodenal artery inferiorly, and runs longitudinally along its course.
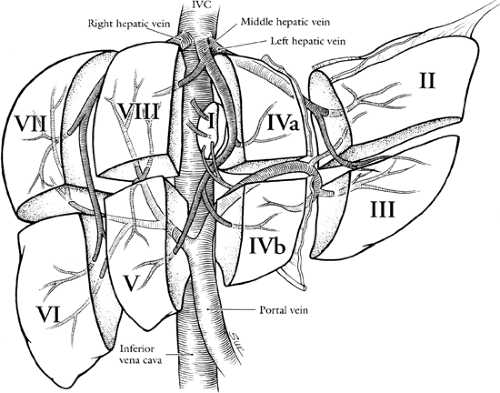
One of the greatest advances in hepatic surgery is the understanding of the segmental anatomy of the liver. The Couinaud system for liver segmental nomenclature is widely accepted (6,7). The liver is divided into longitudinal planes drawn through each hepatic vein to the vena cava and a transverse plane at the level of the main portal bifurcation (Fig. 32.1). The plane of the middle hepatic vein and the primary bifurcation of the portal vein divide the liver into a right and left lobe, this runs from the inferior vena cava to the tip of the gallbladder fossa (also know as the Cantlie line or portal fissure). The secondary portal bifurcations on the right and left give rise to four sectors (also sometimes called segments or sections). The segments are then numbered clockwise in a frontal plane, beginning with the first segment, historically called the caudate lobe. On the right side, this produces the anterior and posterior sectors that are split by the plane of the right hepatic vein. The tertiary branches on the right supply four segments, two in each sector. On the left, the ascending branch gives off recurrent
branches to the medial sector (8), whereas the left lateral sector is supplied by separate branches supplying segments II and III. Segment I, the caudate lobe, receives blood supply from both the left and right portal pedicles; bile ducts from segment I also drain into the right and left hepatic ducts.
branches to the medial sector (8), whereas the left lateral sector is supplied by separate branches supplying segments II and III. Segment I, the caudate lobe, receives blood supply from both the left and right portal pedicles; bile ducts from segment I also drain into the right and left hepatic ducts.
The application of surgical segmental anatomy to axial radiologic imaging (computed tomography [CT] or magnetic resonance imaging [MRI] studies) is critical in evaluating the resection potential of hepatic tumors. Reading axial studies should begin with the identification of the hepatic vein insertions into the vena cava and the plane of the portal bifurcation. Any segments cephalad to the portal vein bifurcation are VII, VIII, IVA, or II. Those caudal to the portal vein bifurcation are VI, V, IVB, and III.
Staging—Clinical Systems
Most patients with HCC present with advanced disease and thus are not candidates for curative therapy. A significant proportion of the remaining patients have coexistent, severe liver disease, including cirrhosis and chronic active hepatitis, and they may not have adequate functional hepatic reserve to tolerate hepatic resection. As a result, there are multiple clinical staging systems for HCC. These systems represent attempts to standardize the initial therapy for HCC. Selection of the most appropriate candidates for surgical resection, liver transplant, or definitive tumor ablative therapy is the ultimate goal. The most widely accepted clinical staging systems include Okuda, Barcelona Clinic Liver Cancer (BCLC), Cancer of the Liver Italian Program (CLIP), and Model for End-Stage Liver Disease (MELD).
Okuda Staging System
A group of Japanese patients with HCC was used to devise this system. It takes into account physiological liver function parameters and tumor characteristics. Liver function parameters include measurement of serum albumin and bilirubin. The other two parameters are the presence of ascites and the tumor size or volume in relation to the hepatic parenchyma (Table 32.1). Each criterion is marked as either positive or negative, depending on the value. A serum albumin of <3 g/dL is “+”. A serum bilirubin >3 mg/dL is “+”. Clinically detectable ascites is “+”. Tumor size of >50% of the largest cross-sectional area of the liver is “+”.
The Okuda stage is determined by the number of positive criterion. The Okuda stage I patient has no positive findings, Okuda stage II has one or two positives, and Okuda stage III has three or four positives. In the original series of 850 patients describing the clinical scheme for staging, patients with Okuda stage I disease who underwent resection displayed a median survival of 25.6 months, whereas Okuda stage II patients treated with hepatic resection had a 12.2-month median survival. Medically treated Okuda stage I patients fared worse with median survival of 9.4 months. Compared to no treatment, Okuda stage II and III patients fared better with medical therapy (stage II: 1.6 vs. 3.5 months, stage III: 0.7 vs.
1.6 months). A majority of these patients presented with advanced disease (>80%), either based on tumor or hepatic function (9). This staging system has been found to be most reliable and reproducible in advanced cases of HCC (10,11).
1.6 months). A majority of these patients presented with advanced disease (>80%), either based on tumor or hepatic function (9). This staging system has been found to be most reliable and reproducible in advanced cases of HCC (10,11).
Table 32.1 Okuda Staging System | ||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| ||||||||||||||||||||
The Okuda system does not provide stratification for small (≤2 cm) tumor size, tumor multifocality, or vascular invasion. Each element has individually been shown to carry significant prognostic value in early HCC (12,13,14). Another criticism of the Okuda system is the heterogeneity of the initial study group. Patients eligible for resection are a more homogenous group based on the clinical parameters used in this system and important differences are not elucidated by the Okuda system. As a result, the Okuda staging system is not typically employed to determine patient eligibility for treatment with curative intent.
Barcelona Clinic Liver Cancer
Developed as a clinical staging system, the BCLC uses variables from tumor stage, liver function (including Child-Pugh classification) (Table 32.2), patient performance status, and symptoms to derive a stage applicable to a treatment algorithm. Early HCC is classified as stage A in the BCLC. This stage includes small solitary tumors and small multinodular tumors in patients with good hepatic reserve and good performance status. Depending on the specific details of each patient’s clinical situation, treatment recommendations shift for different groups within stage A BCLC (Table 32.3). Stage B patients are considered to have large, multifocal disease in a background of good performance status and at least moderate hepatic reserve. The BCLC group recommends chemoembolization alone for stage B. Stage C, also called advanced HCC in this system, represents patients with vascular invasion and/or extrahepatic disease in the setting of diminishing performance status and moderate hepatic reserve. Patients with stage C are offered either a clinical trial with novel agents or supportive care by the authors of the BCLC. Terminal stage HCC, as defined by BCLC, is stage D, and denotes any patient with poor performance status and poor hepatic reserve (Child-Pugh C). These patients are offered symptomatic, supportive care (15,16).
At least two retrospective studies have shown the BCLC staging classification to provide better prognostic data than the other systems outlined in this chapter. Marrero et al. reported a retrospective review of 239 patients from the United States with cirrhosis and HCC. They found in their mix of heterogenous patients that BCLC staging had the best interstage survival predictability with low intrastage variability (17). A similar retrospective study in European patients by Cillo et al. also found BCLC to have better interstage discriminatory survival predictions. This was especially true for the early HCC surgically resected patients (BCLC stage A1) who had a 74% 5-year survival rate. Disappointingly, 5-year survival plummeted to 17% for the remainder of stage A patients (A2–4) (18).
Table 32.2 Child-Pugh Stage Classification | |||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| |||||||||||||||||||||||||||||||
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree