Fig. 23.1
Hepaticojejunostomy stenosis following early repair of a bile duct injury during laparoscopic cholecystectomy in a 27-year-old women. This patient was successfully managed using repeated sessions of percutaneous dilatation. a Percutaneous cholangiography. b Magnetic cholangiography
Incidence and Risk Factors According to the Clinical Context
Since creation of HJ may be required in various surgical situations, both incidence and risk factors of HJ strictures widely vary according to the clinical context (Table 23.1).
Table 23.1
Incidence and risk factors of hepaticojejunostomy strictures according to the clinical situation
Indication for HJ | Incidence of strictures (%) | Risk factors |
|---|---|---|
Bile duct injury | 5–22 | Sepsis during repair |
Absence of bile duct dilatation | ||
Postoperative biliary leakage | ||
Liver transplantation (LT) | ||
Deceased donor | 2–21 | Primary sclerosing cholangitis |
Living donor | 6–22 | Graft related factors: |
Steatosis, Prolonged cold ischemia time, donor age > 50 years | ||
Technical factors: | ||
Biliary anatomical variation, small ducts, no microsurgical repair | ||
Postoperative complications: | ||
Biliary leakage, arterial thrombosis, CMV infection | ||
Pancreatic head resection | 2.6 | – |
Choledochal cyst excision | ||
Children | 0–6 | Type Iva cysts |
Adults | 5–24 | Short duration of symptoms |
Large-sized cysts | ||
Age > 10 years | ||
Iatrogenic Bile Duct Injury
Since the description of the Hepp and Couinaud biliary-enteric anastomosis using the extrahepatic left hepatic duct [14], HJ has remained the standard procedure in the surgical management of most postcholecystectomy bile duct injuries. In this setting, HJ anastomotic strictures nevertheless occur in 5–22 % [15–19] of the patients. The absence of bile duct dilatation has long been incriminated as the most prominent risk factor for the development of HJ stricture following bile duct injury repair [16], leading some authors to either postpone the intervention until bile duct dilatation was obtained or to routinely use transanastomotic stents on non-dilated bile ducts [20]. However, several studies have highlighted that early repair achieved similar results as delayed biliary reconstruction provided that the procedure was performed by a specialist hepatobiliary surgeon [21]. This clearly emphasizes the need for early diagnosis and referral to a specialized HPB unit. Other risk factors for the development of HJ stricture include the presence of biliary peritonitis at repair [22] and postoperative complications following the repair, especially biliary leakage [16], which both intuitively increase the risk of postoperative inflammatory stenosis. Finally, the impact of an associated right hepatic arterial injury in the occurrence of anastomotic stricture is still a matter of ongoing debate [15, 21, 22], with several arguments suggesting a role of arterial injury in favoring ischemia and retraction of the bile ducts and others supporting a rapid revascularization through the anastomosis. In our experience, routine CT scan with vascular reconstruction is always performed to preoperatively assess the arterial vascularization. Similarly, we believe that both operative evaluation of the biliary vascularization and confection of high anastomoses may help to prevent postoperative strictures. Finally, we consider that the existence of an associated vascular injury should probably lead to considering early repair with the utmost caution.
Liver Transplantation (LT)
In deceased donor liver transplantation (LT), HJ is more and more restricted to a limited number of situations including large disparity in size between the recipient’s bile duct and the donor’s bile duct, liver retransplantation, LT for primary sclerosing cholangitis, and biliary atresia. In these situations, the incidence of HJ strictures ranges from 2 %, in the case of liver retransplantation [23], to 21 % in patients with PSC [24]. In this latter setting, it has been recently suggested that duct-to-duct anastomosis (DDA) provided better long-term functional results than HJ [24] without increasing the risk of disease recurrence [25, 26], supporting that it should be probably preferred over HJ. In living donor LT, no randomized study has yet documented the superiority of DDA over HJ. Hence, HJ is still performed in 20–40 % of the cases [27, 28]. In this latter setting, biliary anastomotic strictures represent the Achilles heel of these procedures with reported rates ranging from 6 to 22 % [29, 30]. The occurrence of HJ strictures may be the consequence of: (1) impaired graft quality as evidenced by increased rates of HJ strictures with significant steatotic grafts [31, 32] with prolonged cold ischemia time [33] or grafts from donors aged > 50 years [30]; (2) technical factors including biliary anatomical variations [31] requiring > 1 biliary anastomosis [30] and small donor right or left bile ducts; [31]; and (3) postoperative complications, mainly biliary leakage [30, 34], hepatic artery thrombosis [35], CMV infection [35], and acute cellular rejection [33]. Obviously, prevention of HJ strictures in patients undergoing LDLT may essentially be achieved by improving the selection of the grafts with the systematic use of preoperative donor liver biopsy but also with refinements in surgical technique. In this latter setting, Lin et al. have emphasized the value of routine microsurgical biliary reconstruction in decreasing the number of anastomotic strictures regardless of both types and number of ducts [27].
Pancreatic Head Resection
Biliary complications following pancreatic head resection are often neglected and overlooked by those involving the pancreatic anastomosis. Hence, only one study has to date specifically examined the incidence of biliary strictures after pancreaticoduodenectomy (PD) [3]. In this large single-center study analyzing 1595 patients undergoing PD over 8 years, 42 (2.6 %) patients experienced HJ stricture and median time for stricture occurrence was 13 (median: 1-98) months. No significant risk factor for the development of strictures was observed with only marginal influence of preoperative biliary drainage and no impact of either common bile duct size or postoperative biliary leakage. Interestingly, the rates of strictures were also strictly similar in patients operated on for benign and malignant disease. In this latter context, less than 10 % of the patients were found to have recurrent neoplastic disease involving the bilioenteric anastomosis. Of these, none were operated on for pancreatic or ampullary carcinoma supporting that development of a biliary stricture in these patients is usually benign. However, all patients with malignant anastomotic strictures carried a diagnosis of cholangiocarcinoma. This result, which is in line with the reported 16 % rate of tumor recurrence at the proximal stump in patients operated on for extrahepatic cholangiocarcinoma [36], suggests that anastomotic tumor recurrence should be systematically ruled out in this subset of patients.
Choledochal Cyst
In the long-term follow-up of patients undergoing choledochal cyst excision, the development of postoperative HJ anastomotic stricture widely varies according to the age of the patient at the time of surgery. Indeed, the rates of HJ strictures range from virtually 0 to 6 % in children [37, 38] when the anastomosis is performed on the hepatic hilum, while it may reach up to 24 % in adults [39]. This finding is probably related to the fact that inflammation of the cyst wall is mild in children under 10 years of age and more severe in older children and adults, likely resulting from severe histological damage to the common hepatic duct used for a bilioenteric anastomosis [40]. Other risk factors for the development of HJ after choledochal cyst excision include shorter duration of symptoms [39], increased size of the cyst [39], and type IVa cysts [40] where inflammation is associated with histological damage of the common hepatic duct after HJ and may lead to severe scaring at the bilioenteric anastomosis [40]. Altogether, these results suggest that the balance between the risks of malignant transformation and the risks of invalidating symptoms following HJ stricture, especially in adults with type IVa cysts, should lead to cautious consideration of surgery on a case-by-case basis rather than on a systematic operative approach basis. In this situation, definition of a subgroup of patients at low risk of malignant transformation would probably allow avoiding unnecessary procedures at extremely high risk of postoperative complications.
Therapeutic Options
In patients with HJ strictures, a multimodal and gradual management with repeated treatment sessions and a combination of several approaches is often required (Fig. 23.2). Treatment options, which include conservative management with endoscopic, percutaneous transhepatic, or transjejunal balloon dilatation and surgery from revisionary HJ to LT, depend on the clinical situation and the existence of associated complications. In this setting, the Terblanche classification [41], which was designed for the assessment of biliary repair following bile duct injury, stratifies the functional results of HJ into IV grades (Table 23.2) and may be of value in the analysis of the efficacy of the management of these strictures.
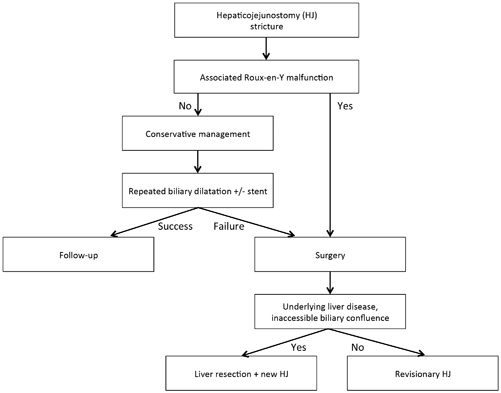

Fig. 23.2
Proposed management of patients with hepaticojejunostomy strictures. In patients with isolated HJ strictures, first-line treatment should be as much conservative as possible. Surgery should remain a second-line treatment after failure of well-conducted conservative management or in rare cases of associated Roux-en-Y malfunction
Table 23.2
Classification of the functional results of hepaticojejunostomies. (Derived from Telbranche et al. [41])
Grade I. No biliary symptoms |
Grade II. Transitory symptoms, currently no symptoms |
Grade III. Clearly related symptoms requiring medical therapy |
Grade IV. Recurrent stricture requiring correction or related death |
Conservative Management
Choice of the Approach
The percutaneous approach remains the approach of choice with reported therapeutic success rates reaching 90–100 % [42, 43]. In this setting, a multistep strategy is generally undertaken. The first step usually consists in transhepatic cholangiography and external catheter drainage. A single-puncture technique is used whenever direct insertion of a thin wire offers a suitable approach to the biliary system. Otherwise, a common double-puncture technique is performed. Percutaneous transhepatic tracts are created to ensure complete drainage of all excluded territories. Once the bilioenteric stricture has been passed, insertion of one or several external catheters allows drainage of the entire biliary tree above the stricture. When present, small biliary tract stones might be removed using irrigation with saline solution or may be pushed forward through the bilioenteric anastomosis [5]. On the other hand, large stones might be removed after percutaneous electrohydraulic lithotripsy under cholangioscopic guidance [5, 42]. The second step is usually performed between 3 and 7 days later. An angioplasty balloon catheter is inserted across the stenosis and inflated gradually. Thereafter, stenting is achieved using an internal–external biliary drainage or wall-stent placement. Control cholangiography with catheter exchange and complementary dilatations are performed every 6 weeks. When no residual stenosis is observed on at least two consecutive sessions, the catheter is removed and the patient is followed regularly to detect any recurrent stricture.
Recently, several teams have reported their results using endoscopic retrograde balloon dilatation. This approach, which may facilitate both multiple stent placement [7, 9] and use of lithotripsy, however, currently only provides success rates of 70 % cases using single-balloon enteroscope [7]. Even though endoscopy may be facilitated with the use of short-limb Roux-en-Y [44] reconstruction or positioning of the Roux-en-Y loop on the duodenum, it should be restricted to experienced centers in the setting of therapeutic evaluation. The percutaneous transjejunal approach represents a valuable alternative to the endoscopic approach with satisfactory long-term results but is also restricted to very few experienced centers [12, 13]. Finally, the “rendez-vous” technique, which combines both endoscopic and percutaneous approaches, may be useful in complex situations. However, in a setting of HJ stricture, this strategy remains clearly marginal with only limited reported experience [45, 46].
To Stent or Not to Stent?
The rationale of using metallic wall-stent would be to allow limiting the number of procedures and decrease hospital stays [47]. However, despite initial promising results and high primary technical success rates [48, 49], long-term results of benign biliary stricture treatments by metallic stents have been tempered by high rates of late re-occlusion [50]. On the other hand, retrievable covered stent seems to be a good alternative to shorten treatment duration compared to interposition of an internal–external catheter. However, the risk of branching bile duct occlusion limits its use in the setting of living donor LT recipients owing to high frequency of complex biliary anastomotic strictures.
Periprocedural Management
Preanesthetic consultation and routine blood tests including a coagulation profile are systematically required. Similarly, the vast majority of the patients have contaminated bile, and it is mandatory to systematically start antibiotherapy prophylaxis before the procedure in order to prevent the occurrence of severe septic complications during manipulation of the bile ducts. Antibiotics are generally continued for at least 2–5 days following the procedure. Since most of the patients will require several therapeutic sessions, it is important to adapt the antibiotics to the microbiological findings of previous interventions. After the procedure, occurrence of blood in the drainages should lead to immediate elimination of vascular complications such as active hemorrhage, hematoma, or pseudo-aneurism on CT scan. Similarly, in patients with external drainage, tubes should be flushed daily with 5–10 ml of saline to ensure adequate bile outflow and bile loss should be rigorously compensated. If occlusion is suspected in the absence of bile outflow, proper fixation of the drainage should be verified and tubes may be flushed with 5–10 ml of saline. In the persisting absence of bile outflow, radiological assessment of the drainage with standard X-ray, CT scan, or percutaneous cholangiography should be undertaken. Finally, when the internal–external drainage has been placed, occurrence of moderate fever or mild elevation of hepatic enzymes is common after first occlusion of the external part of the drain and should lead to its reopening. After a few days, a new attempt might be undertaken. In case of recurring symptoms, control of catheter placement should be undertaken.
< div class='tao-gold-member'>
Only gold members can continue reading. Log In or Register to continue
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree







