Chapter 83 Hepatic artery embolization and chemoembolization of liver tumors
Overview
Primary and secondary liver tumors cause tremendous neoplastic morbidity and mortality. Hepatocellular carcinoma (HCC) is the fifth most common cancer and the third most common cause of cancer-related death in the world (Bosch et al, 2005). The highest incidences of HCC are found in Eastern Asia and sub-Saharan Africa, where hepatitis B is endemic; however, the incidence of HCC has been increasing steadily in the United States and Western Europe, largely owing to increased prevalence of viral hepatitis C in the past 2 decades (El-Serag et al, 2003). The majority of metastatic cancers involve the liver at some point as disease progresses. Despite the presence of extrahepatic spread, many patients with liver involvement die as a consequence of local tumor growth and hepatic parenchymal destruction.
Basic Principles of Hepatic Arterial Embolotherapy
Blood Supply of Liver Tumors
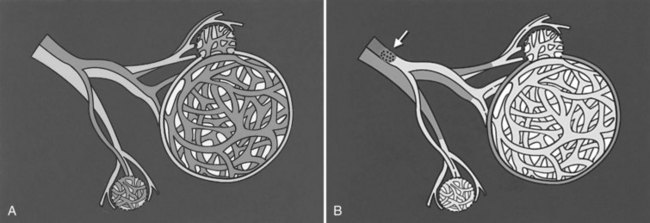
The basic physiologic principle that makes HAE feasible in patients with liver tumors is the dual blood supply to the liver (see Chapter 1B). The portal vein provides more than 75% of the blood flow to the normal hepatic parenchyma and is the primary trophic blood supply. Conversely, most of the blood supply (90% to 100%) to liver tumors comes from the hepatic artery (Breedis & Young, 1954), thus embolization of tumor-feeding arteries leads to selective ischemic damage of the tumor, while sparing the normal liver parenchyma, mainly supplied by the portal vein. Moreover, the pharmacokinetic advantage of locoregional drug administration enhances the theoretic benefit. For example, hepatic drug exposure has been estimated to be double for doxorubicin, 7 times greater for cisplatin, 8 times greater for mitomycin, 10 times greater for 5-fluorouracil, and up to 400 times higher for 5-fluorodeoxyuridine, when these were given through the hepatic artery rather than through the systemic veins (Ensminger & Gyves, 1983; see Chapter 86).
Hepatocarcinogenesis is a multistep process that causes gradual arterialization in blood supply to tumors (Pang & Poon, 2006), therefore the blood supply of liver tumors can be variable, according to the carcinogenetic stage of the tumors. For example, encapsulated nodular HCC is almost exclusively supplied by the hepatic artery, and well-differentiated HCC and the extracapsular infiltrating edge of advanced HCC can be supplied by the portal vein or both the portal vein and the hepatic artery (Goseki et al, 1995). Similar observations have been made in metastatic liver tumors; in its early stage, less than 200 µm is supplied almost exclusively by sinusoidal blood. As the tumor grows, its blood supply becomes progressively arterialized, but even in the advanced stage, most liver metastases still have a distinct portal blood supply (Strohmeyer et al, 1987); therefore early stage tumors and some fraction of advanced tumors may be resistant to hepatic arterial embolotherapy.
Chemoembolization
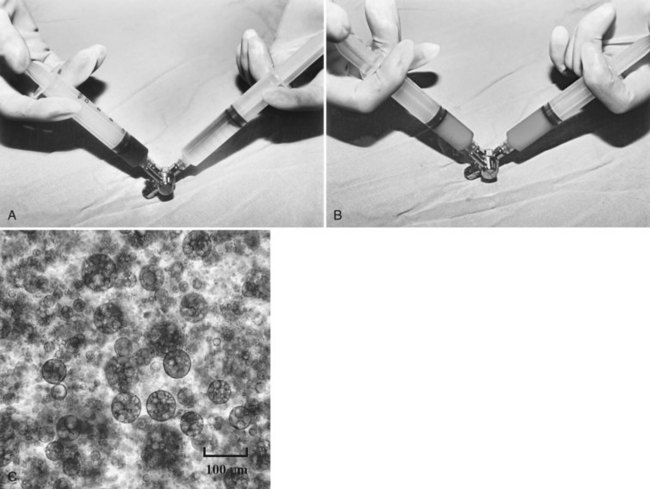
In the past 3 decades, many papers have been published describing a myriad different techniques for performing HAE; combined with intraarterial chemotherapy (chemoembolization), HAE has been paramount among them as the most widely used procedure. The goal of chemoembolization is to combine the effects of targeted tumor ischemia by embolization with intraarterial chemotherapy. To date, the most common sole-agent chemotherapeutic drug has been doxorubicin, followed by cisplatin, epirubicin, mitoxantrone, mitomycin C, and a chemical conjugate of a synthetic copolymer of styrene maleic acid and neocarcinostatin (SMANCS) (Marelli et al, 2007). The chemotherapeutic drug is dissolved in water or water-soluble contrast agent and is then mixed with iodized oil (Lipiodol; Laboratoire Guerbet, France) and administered as an emulsion (Fig. 83.1). When injected into the hepatic artery, iodized oil is trapped selectively in the tumor because of the hemodynamic difference between the tumor and normal liver parenchyma and presumably the absence of Kupffer cells in the tumor (Kan et al, 1993; Nakajo et al, 1988). Thus mixing iodized oil with a chemotherapeutic drug allows slow release of the chemotherapeutic drug over a period of 6 to 12 weeks (Raoul et al, 1992). In contrast, in the normal liver parenchyma, the iodized oil does not occlude the hepatic artery; rather it accumulates in the peripheral portal vein through arterioportal communications and subsequently passes through sinusoids into the systemic circulation (Chung et al, 1993). HAE after infusion of chemotherapeutic drugs increases drug dwelling time in the tumor by slowing the rate of efflux from the hepatic circulation. Furthermore, ischemic damage by embolization potentiates absorption of chemotherapeutic drugs by impeding the function of transmembrane pumps in tumor cells (Yu & Keeffe, 2003).
After infusion of the chemotherapeutic drug and iodized oil mixture, tumor-feeding peripheral hepatic arteries are embolized. Proximal arterial occlusion is not desirable, because it will not only induce development of intrahepatic and extrahepatic collateral vessels, it will also preclude repeat procedure. Thus it is important to select embolic materials of the proper size. The optimal size should be small enough to reach and occlude the capillaries to the tumor but bigger than the arteriovenous shunt and peribiliary plexus, to avoid the risk of pulmonary embolization and bile duct necrosis. To date, gelatin sponge particles have been the most frequently used agent, but polyvinyl alcohol (PVA) particle (Ramsey et al, 2002), absolute ethanol (Matsui et al, 1993), starch microspheres (Maluccio et al, 2008), cyanoacrylate (Berghammer et al, 1998), and even autologous blood clot (Gunji et al, 2002) have also been used.
Gelfoam is used as 500- to 1000-µm cubes, which only occludes the artery temporarily, with recanalization taking place within 2 weeks (Coldwell et al, 1994). It was proved not to cause serious hepatic damage in patients with good hepatic functional reserve (Kan et al, 1993; Sonomura et al, 1997); however, gelfoam powder should not be used, as it may cause biliary stenosis and biloma resulting from embolization and necrosis of small arteriolar branches, which may cause biliary damage (Makuuchi et al, 1985). PVA particles cause a permanent or semipermanent arterial occlusion and can achieve more distal obstruction because of their smaller size (50 to 250 µm in diameter; Coldwell et al, 1994). Autologous blood clot achieves the same temporary artery occlusion as a gelatin sponge. Because the clot is lysed faster after embolization, there might be less chance of arterial thrombosis after several sessions of chemoembolization (Gunji et al, 2002). In a recent comparative nonrandomized study, no difference in patient survival was found between chemoembolization using gelatin sponge particles and that using PVA particles (Brown et al, 2005).
It is important to evaluate tumor-related arteriovenous shunt for safe procedures. In patients with HCC, angiographic incidence of shunting has been reported in 31.2%, shunting into the portal vein has been reported in 28.8%, and hepatic vein shunting has been reported in 2.4% (Ngan & Peh, 1997). The more liver parenchyma is occupied by the tumor, the more arteriovenous shunts exist; these are associated with poor prognosis (Granov et al, 1992) and are frequently problematic in chemoembolization of liver tumors (Izaki et al, 2004). Severe arterioportal shunt can cause hepatofugal portal flow, ascites, and variceal bleeding. In patients with a prominent arteriovenous shunt, embolization of the shunt is recommended prior to HAE (Huang et al, 2004). After embolization for massive arterioportal shunt, hepatofugal portal flow may convert to hepatopetal flow, and performance status and ascites may be improved (Izaki et al, 2004).
Patient Selection
Chemoembolization is indicated for the treatment of unresectable hypervascular liver tumors, which receive more blood supply from the hepatic artery than normal liver parenchyma, which makes the tumor suitable to HAE. The hemodynamic characteristics of a tumor may be documented on contrast-enhanced ultrasonography (Suzuki et al, 2003), computed tomography (CT) (Tajima et al, 2002b), and magnetic resonance imaging (MRI) (Levy, 2002).
The most common indication of chemoembolization is unresectable HCC. The determination of resectability of HCC should be based on either the extent of tumor involvement, underlying liver function, or a combination of these (see Chapters 2 and 90A). The majority of patients with HCC have underlying liver cirrhosis, which often requires a larger remnant liver after surgery to maintain hepatic function than in patients without underlying liver disease; therefore tumors that might be resectable in patients with normal liver parenchyma may not be resectable in patients with cirrhosis. Hepatic dysfunction also acts as a limitation in selecting patients for chemoembolization. Patients with poor hepatic function are not likely to tolerate extensive arterial embolization, because their livers are more dependent on arterial blood supply than normal liver, and moreover, patients with severe cirrhosis are more likely to die of their underlying liver disease than of HCC. Thus chemoembolization is typically recommended in patients with reasonably preserved liver function (Child-Turcotte-Pugh [CTP] class of A or B) and performance status (Eastern Cooperative Oncology Group [ECOG] 0 or 1). In general, patients with class C cirrhosis should not undergo chemoembolization as a primary treatment.
The extent of tumor is another important consideration in selecting patients for chemoembolization. Several methods have been proposed to weigh both hepatic function and the extent of tumor. In Okuda staging, patients are given points for presence of ascites, serum bilirubin and albumin levels, and the extent of tumor, and the patient is staged as I, II, or III (Okuda et al, 1985). The Cancer of the Liver Italian Program (CLIP) group proposed a more complex scoring system that includes the CTP score, tumor morphology and extension, presence of portal vein thrombosis, and serum α-fetoprotein (AFP) levels. The CLIP score was validated worldwide in multiple clinical trials and was proven to give more accurate prognostic information and greater survival predictive power than the Okuda staging system (CLIP, 2000; Ueno et al, 2001); however, those staging systems for predicting prognosis of patients with HCC did not indicate which patients would benefit from HAE. Recently, the Barcelona Clinic Liver Cancer (BCLC) staging system was developed to allow for the indication of the best therapy for each stage of HCC (Llovet et al, 1999a). In this staging system, chemoembolization is recommended as first-line therapy for intermediate stage HCC (Okuda staging 1 to 2, performance score 0, and large or multinodular disease) and some advanced-stage HCC (Okuda 1 to 2, performance score 1, no extrahepatic disease).
Spontaneous rupture of HCC is an indication for emergency HAE, regardless of underlying liver function (Kirikoshi et al, 2009). Even in patients with advanced liver cirrhosis, nodular HCC showing exophytic growth can be managed safely by selective embolization to prevent tumor rupture without diminishing liver function. Chemoembolization plays additional neoadjuvant roles as a downstaging therapy before resection or as a bridge therapy for patients awaiting liver transplantation (see Chapter 97D).
Although no absolute contraindications to HAE exist, patients with two or three major poor prognostic indicators should not be treated. Generally accepted relative contraindications are extensive tumor involvement of more than 50% to 75% of the liver and class C cirrhosis. Major portal vein invasion by the tumor has been considered another relative contraindication, but this can be safely and effectively managed by an adjustment of the embolization protocol to reduce the amount of chemoembolic agents and the extent of the embolization, especially in patients with a limited parenchymal tumor and adequate liver function (Chung et al, 1995). Recent evidence demonstrates that HAE can be safely performed, even in patients with main portal vein occlusion, if hepatopetal collateral flow is present (Lee et al, 1997; Tazawa et al, 2001; Georgiades et al, 2005). In addition, active gastrointestinal bleeding, refractory ascites, extrahepatic spread, hepatic encephalopathy, and biliary obstruction are also considered relative contraindications. Regardless of the liver tumors, anaphylactoid reaction to contrast media or renal insufficiency, uncorrectable coagulopathy, and severe peripheral vascular disease can preclude embolotherapy.
Procedure
Before any chemoembolization procedure, laboratory tests should be done that include a complete blood cell count, prothrombin time, creatinine levels, and liver function testing. The baseline serum levels of tumor markers should be measured before treatment to monitor changes after treatment. By means of cross-sectional imaging studies, the size and extent of the tumor, its growth pattern (expansile vs. replacing or infiltrating), and macroscopic angioinvasion into the hepatic or portal veins are evaluated. For successful chemoembolization, accurate segmental localization of the tumor is crucial, and cross-sectional images are useful for that purpose (Yoon et al, 2008). In addition, imaging studies of the chest, abdomen, and pelvis are recommended to assess comorbid disease and ensure the presence or absence of metastatic disease.
Patients are fasted overnight and hydrated with normal saline at a rate of 200 to 300 mL/h. Antiemetics are administered intravenously, and patients with contrast allergies receive oral prednisone 1 hour before the procedure. The use of prophylactic antibiotics is controversial except in patients with a bilioenteric anastomosis or biliary stent (Geschwind et al, 2002; Ong et al, 2004; Tarazov, 1995). After infiltration of local anesthetic, the Seldinger technique is used to gain access to the common femoral artery, and initial diagnostic visceral arteriography is performed to determine arterial anatomy to the liver and patency of the portal vein. For complete angiography, all hepatic arteries should be adequately opacified, and all feeding arteries of the tumor should be identified.
Anatomic variations of celiac trunk and hepatic arteries are commonly encountered. With the introduction of multidetector row CT, these anatomic variations can be predicted before the procedure by careful review of the arterial phase scan (Song et al, 2010). Most common hepatic artery variations are the right hepatic artery arising from the superior mesenteric artery and the left hepatic artery arising from the left gastric artery, thus celiac and superior mesenteric arteriography is mandatory to identify the variations. Frequently, additional views in different angles and magnifications and selective contrast injection with use of a microcatheter are necessary to identify small feeding arteries (Yoon et al, 2008). To avoid nontargeted embolization, it is important to recognize the origin of the cystic artery and the right gastric artery, presence of a large falciform artery, and the accessory left gastric artery originating from the left hepatic artery (Song et al, 2006).
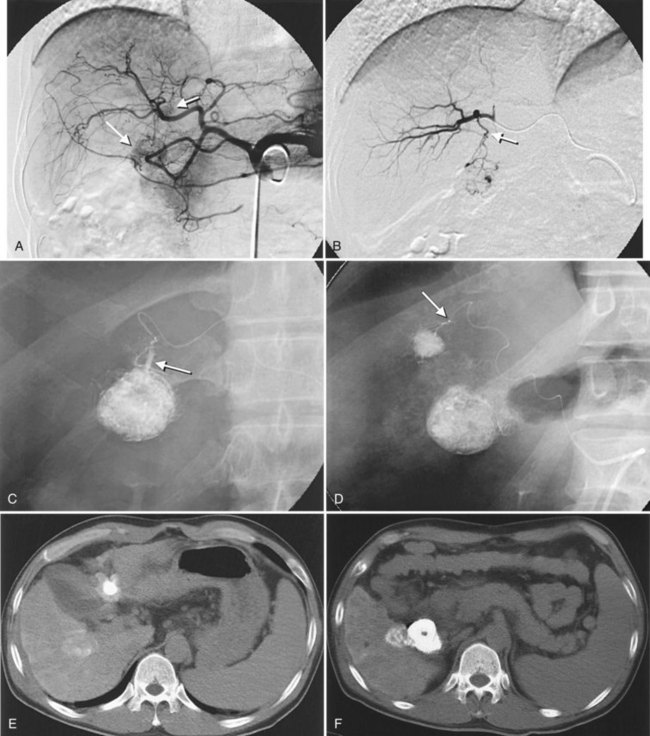
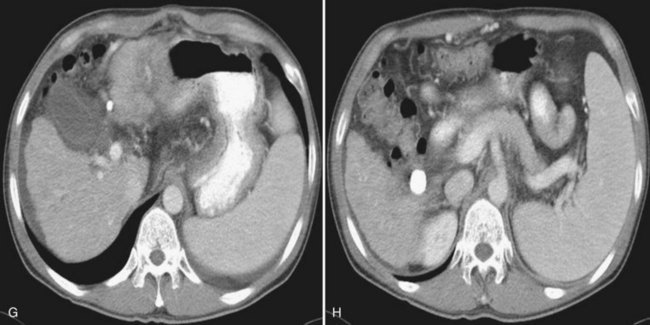
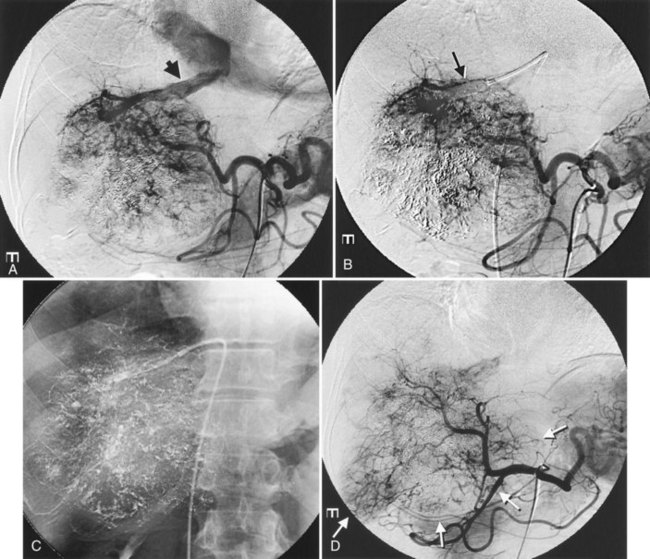
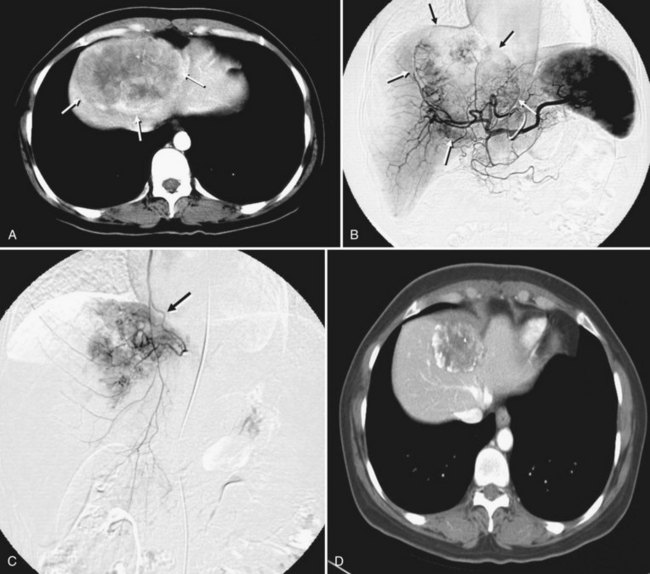
After the catheter is positioned appropriately, the mixture of iodized oil and chemotherapeutic drug is injected (Figs. 83.2 and 83.3). If there is a prominent arteriovenous shunt that precludes chemoembolic agents reaching the tumor vasculature, it is recommended first to embolize the shunt before injection of chemotherapeutic drugs (Fig. 83.4). The dose of iodized oil and chemotherapeutic agent depends on the size and vascularity of the tumor. The generally accepted upper limit of iodized oil is 15 mL. For a small tumor, enough iodized oil to saturate the entire tumor neovasculature could be administered. The end point for the mixture administration is stasis in tumor-feeding arteries or appearance of iodized oil in portal vein branches (see Fig. 83.3). If flow is a persistent in the tumor-feeding arteries after infusion of chemotherapeutic drug, HAE should be performed. Gelatin sponge particles are the embolic agents most frequently used, because they degrade in a few weeks, allowing repeat treatment. PVA particle is another commonly used embolic agent, which causes a more permanent arterial occlusion (Ramsey et al, 2002).
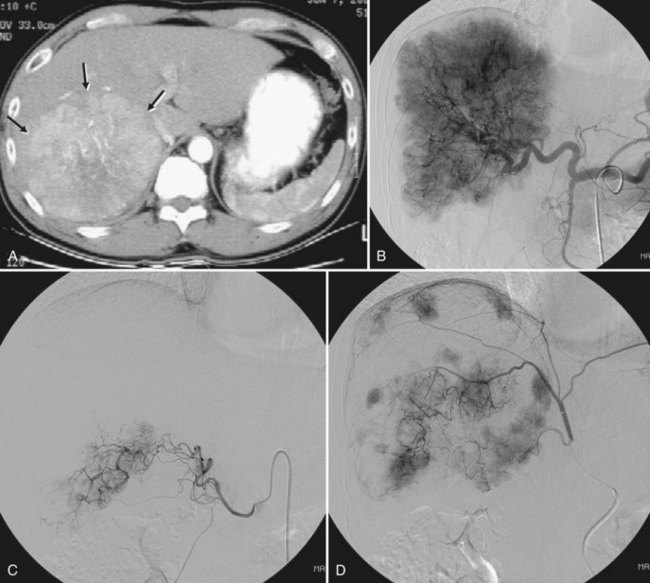
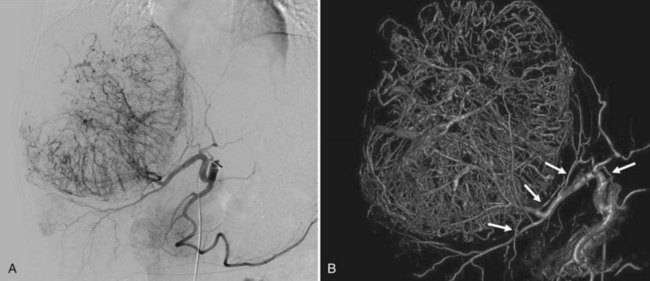
Embolization of extrahepatic collaterals supplying the tumors is crucial to achieve successful outcomes. When a tumor is adjacent to a hepatic bare area or suspensory ligaments, or it invades into an adjacent organ, selective arteriogram of possible extrahepatic arteries should be obtained. With recent advances in CT imaging, those collateral vessels can be detected in preprocedure imaging studies (Kim et al, 2005). Common extrahepatic collaterals include the inferior phrenic artery, omental artery, internal mammary artery, colic branch of superior mesenteric artery, adrenal artery, intercostal artery, renal capsular artery, and gastric arteries (Chung et al, 2006; Kim et al, 2005, 2007, 2009; Won et al, 2003; Figs. 83.5 and 83.6). When the hepatic artery and extrahepatic collaterals supply the tumor, additional chemoembolization of the extrahepatic collaterals can be tried to increase the therapeutic efficacy of chemoembolization. These extrahepatic collaterals can be used as an access route to the tumors in patients with hepatic artery occlusion.
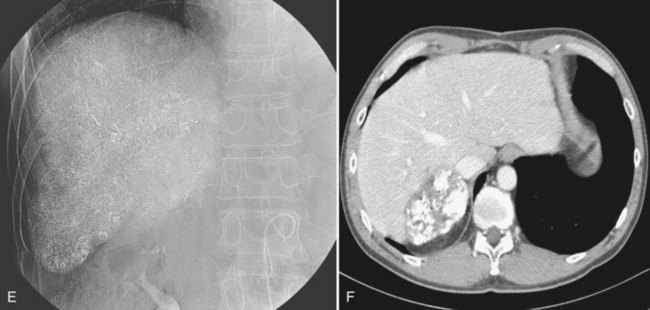
Recently, the use of C-arm cone-beam CT has been increasing. It can provide information about a patient’s vascular anatomy, associated liver parenchyma, and the target lesion that constitutes a substantial improvement over conventional digital-subtraction angiography (DSA) and fluoroscopy (Carlson et al, 2001). C-arm cone-beam CT enables more selective catheterizations, which increases the amount of therapeutic agent delivered to the target area and decreases nontumorous liver parenchymal exposure to the agent (Meyer et al, 2007; Wallace et al, 2008; Fig. 83.7) In addition, confident identification of extrahepatic arteries supplying nontarget organs, such as supraduodenal or retroduodenal arteries, right gastric artery, phrenic artery, and falciform artery, is crucial to avoid nontarget embolization (Kim et al, 2009; Liu et al, 2005). When feeding arteries for a tumor are multiple, the portion of the tumor supplied by a catheterized feeding artery can be estimated, and the chemoembolic regimen can be proportioned accordingly. Iodized oil preferentially accumulated within tumors can be imaged without use of additional contrast media; thus completeness of therapy can be assessed immediately after treatment. If the oil has not accumulated in a portion of the tumor, the operator can search for hepatic or extrahepatic collateral tumor supply (Meyer et al, 2007; Wallace et al, 2007).
Intraarterial lidocaine and intravenous narcotic analgesics are given before the procedure to relieve pain during and after (Lee et al, 2001b). After the procedure, hydration is continued with normal saline. Narcotic analgesics, chlorpromazine, and acetaminophen are additionally supplied for 1 to 3 days as needed. Patients can be discharged once they have resumed adequate oral intake, and intravenous analgesics are no longer required.
Laboratory studies of liver function and serum levels of tumor markers should be repeated 2 to 4 weeks after the procedure. Multiphase contrast-enhanced CT or MRI is recommended 4 to 8 weeks after the procedure to evaluate the efficacy of the treatment and to detect local or remote tumor recurrences. If viable tumor is evident on imaging studies, serum levels of tumor markers are elevated, or both, patients return for another session of embolotherapy according to their hepatic functional recovery. No consensus has been reached on the ideal protocol for repeat procedures, but based on a retrospective comparison, survival rates and patient tolerance seemed to be higher when treatment was repeated, but only when progression of HCC was noted radiographically (Ernst et al, 1999).
Therapeutic Efficacy of Chemoembolization
Hepatocellular Carcinoma
Numerous studies have shown that chemoembolization induces a significant tumor necrosis without negative influence on liver function in patients with preserved liver function. The extent of tumor necrosis has been reported to range from 60% to 100%. With introduction of subsegmental chemoembolization using a microcatheter, the tumor necrosis rate was markedly improved. Matsui and colleagues (1993) reported complete necrosis in about 70% of 100 small hypervascular HCCs less than 4 cm in diameter treated by subsegmental chemoembolization; however, especially in large tumors, even though necrosis may appear macroscopically complete, histologic examination reveals viable neoplastic cells responsible for the high rate of recurrence after treatment. During follow-up the residual tumor nests recover their blood supply, and the tumor continues to grow. Additional treatments may be administered, either at specified intervals or as needed, but no data plainly favors either strategy (Bruix et al, 2004). Several studies have suggested that tumor characteristics lead to favorable response after chemoembolization—including small, encapsulated, expansile, hypervascular tumors—and long-term retention of iodized oil may be seen on CT (Itsubo et al, 2002; Kuroda et al, 1991; Nakamura et al, 1993). Pathologically, trabecular-type HCC showed more prominent necrosis than scirrhous, compact, or well-differentiated HCC (Jinno et al, 1992).
Several case-control and retrospective studies have shown a survival benefit in patients treated with chemoembolization compared with untreated patients or historic control subjects (Aoyama et al, 1992; Bismuth et al, 1992; Bronowicki et al, 1994; Rose et al, 1999). Bronowicki and colleagues (1994) performed a case-control study that stratifed 254 patients according to CTP classification and Okuda staging. The survival rates of the chemoembolization group were significantly higher (64%, 38%, 27%, and 27% at 1, 2, 3, and 4 years, respectively) than those of the conservative management group (18%, 6%, and 5% at 1, 2, and 3 years, respectively). The survival benefit from chemoembolization was not confirmed in early randomized, controlled trials (RCTs) performed in the 1990s (Camma et al, 2002; Groupe d’Etude et de Traitement du Carcinome Hépatocellulaire, 1995; Pelletier et al, 1990). However, two newer RCTs, one from Barcelona (Llovet et al, 2002) and one from Hong Kong (Lo et al, 2002), reported a significant positive impact of chemoembolization on patient survival. This permitted a positive result in a cumulative meta-analysis of all RCTs published from 1978 to 2002; thus, according to the guidelines published by the American Association for Study of Liver Diseases (AASLD) (Bruix & Sherman, 2005) and the European Association for the Study of the Liver (EASL) (Bruix et al, 2001), chemoembolization is recommended as first-line noncurative therapy for nonsurgical patients with large or multifocal HCC who do not have vascular invasion or extrahepatic spread. The improvement in survival in treated patients ranges from 20% to 60% at 2 years (Bruix et al, 2004
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree